-
犬弓首蛔虫Toxocara canis,T. canis是犬常见的胃肠道寄生线虫,其感染性幼虫可以感染人和多种动物[1],是引起弓首蛔虫病的主要病原.该病的流行和分布极为广泛,据资料报道其感染率为1.4%~64.7%[2-3]. T. canis感染人和其他非特异性宿主可引起内脏幼虫移行症(Visceral larva migrans,VLM)、眼睛幼虫移行症(Ocular larva migrans,OLM)和神经幼虫移行症(Neurological larva migrans,NLM),造成严重的病理综合征[4-7].
卵黄原蛋白(vitellogenin,Vg)是脂质转运蛋白家族的成员之一[8-9],普遍存在于卵生非哺乳动物体内[10].不同来源的卵黄原蛋白具有3个保守功能结构域:位于N端的功能结构域(Vitellogenin,LPD_N)、未知结构域(domain of unknow function,DUF1943)和位于C端的vWD结构域(von-Willebrand factor type D domain,vWD)[11-13]. Vg主要是在卵巢外组织中合成,分泌进入循环系统到达卵巢,通过受体介导的内吞作用进入卵母细胞,为正在发育的胚胎或幼虫提供营养物质[14-15].此外,Vg还具有免疫防御、粒子载体、抗氧化活性、筛选内分泌干扰物、调查环境毒理和污染等[16-19]作用.目前,对于昆虫、甲壳动物和脊椎动物的Vg研究较多[20-21],但对于寄生虫卵黄原蛋白基因的研究相对较少.
本研究拟运用分子生物学技术,克隆犬弓首蛔虫Tc-duf1943,构建pET-28a(+)/duf1943原核表达载体,以期能在大肠杆菌E. coli中得到表达,优化其表达条件,为进一步研究TcDUF1943的生物学功能奠定基础.
全文HTML
-
T. canis采自西南大学荣昌校区动物医院某患病犬,由本实验室分离鉴定,液氮速冻并保存.
胶回收试剂盒、M-MLV反转录酶、SYBR® Premix Ex Taq TM Ⅱ、原核表达载体pET-28a(+)及限制性内切酶Bam H Ⅰ、Hind Ⅲ均购自TaKaRa公司;质粒提取试剂盒购自北京全式金生物技术有限公司.
-
根据T. canis的转录组数据(GenBank:JPKZ01002217),使用软件Primer premier(Version 5.0)分别对Tc-duf1943及18S rRNA基因进行引物设计(表 1),并由南京金斯瑞生物科技有限公司合成.下划线分别为限制性内切酶Bam H Ⅰ和Hind Ⅲ的识别位点.
-
采用Trizol试剂分别对T. canis雌虫和雄虫成虫、雌虫生殖道、肠道、体壁及雄虫生殖道、肠道、体壁的总RNA进行提取,采用核酸测定仪检测总RNA的浓度和纯度,以提取的T. canis总RNA为模板,按M-MLV反转录试剂盒说明合成cDNA.
-
以合成的cDNA为模板进行PCR扩增.反应条件为94 ℃预变性3 min;94 ℃变性30 s、56 ℃退火30 s、72 ℃延伸1 min,40个循环;最后72 ℃延伸10 min.扩增产物进行1%琼脂糖凝胶电泳分析,用凝胶成像系统扫描并记录结果.
-
利用TransGen Biotech公司的DNA胶回收试剂盒切胶回收PCR产物,按体系将目的片段与pMDTM19-T载体进行连接:在0.2 mL离心管中加入5 μL的Ligation SolutionⅠ,3 μL的PCR纯化产物,1 μL的pMDTM19-T Vector,1 μL的ddH2O,16 ℃连接4 h.将上述连接产物转化至30 μL的DH5a感受态细胞,涂布于含IPTG(24 mg/mL)和X-gal(20 mg/mL)的LB/Amp琼脂平板中,于37 ℃恒温培养箱正置30 min,再倒置培养12~16 h.
-
将测序正确的重组质粒与pET-28a(+)表达载体经限制性内切酶Bam H Ⅰ,Hind Ⅲ双酶切,回收酶切过后的目的基因与pET-28a(+)质粒,于反应体系中进行连接,重组表达载体经热激法转化E. coli DH5α感受态细胞,经LB/ Kan平板初筛,挑取单菌落于LB/ Kan液体培养基中,220 r/min振荡培养12 h,提取质粒,进行PCR、双酶切及测序鉴定.
-
提取重组质粒,转化E. coli BL21(DE3)感受态细胞,挑取单菌落接种于LB/ Kan液体培养基,37 ℃ 220 r/min振荡培养,当OD600值达到0.6~1.0时,加入0.4 mmol/L的IPTG诱导4 h,将菌液于4 ℃,8 000 r/min进行离心,收集菌体,超声波破碎,分离上清和沉淀,并进行SDS-PAGE电泳检测,鉴定重组蛋白的相对分子质量、表达形式及表达量.检测到目的蛋白后,再分别进行IPTG浓度梯度和时间梯度的优化.
-
取大量诱导表达后的菌液,4 ℃ 6 000 r/min离心20 min,收集菌体,超声波破碎,离心收集目的蛋白,用8 M尿素溶解包涵体,4 ℃ 8 000 r/min离心30 min,上清经Ni-NTA亲和层析柱纯化,SDS-PAGE检测纯化效果,-80 ℃保存备用.
1.1. 材料
1.2. 方法
1.2.1. 引物的设计与合成
1.2.2. 总RNA的提取与反转录
1.2.3. 目的基因的PCR扩增
1.2.4. PCR产物的克隆
1.2.5. 原核表达载体的构建
1.2.6. 诱导表达和SDS-PAGE分析
1.2.7. 重组蛋白的纯化
-
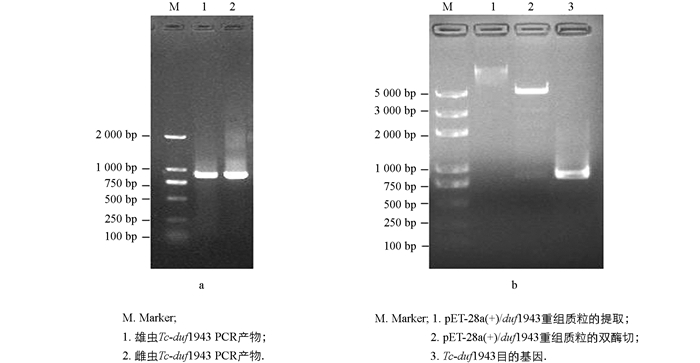
经PCR扩增,1%琼脂糖凝胶电泳结果显示在850 bp左右处可见清晰的条带,大小与预期的目的片段相符(图 1a).将Tc-duf1943亚克隆到pET-28a(+)载体,经双酶切鉴定,结果表明目的片段成功连接到pET-28a(+)载体上(图 1b).
-
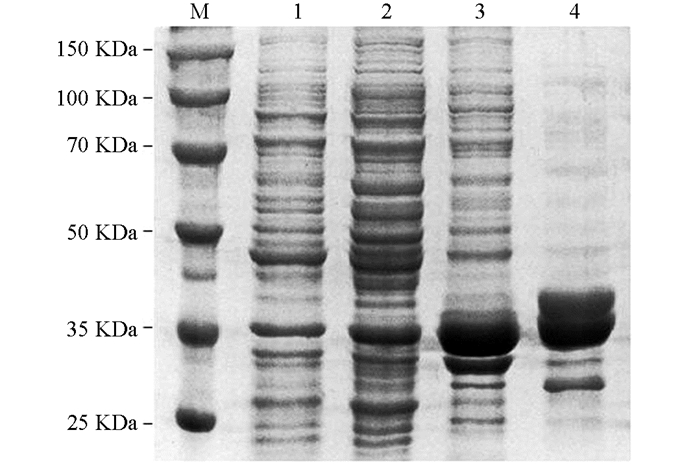
将阳性重组质粒转化E. coli BL21(DE3),经IPTG体外诱导表达,产物以包涵体的形式存在,大小约为3.5×104,与理论预测值相符(图 2);对诱导条件进行优化,结果表明37 ℃,IPTG浓度为0.4 mmol/L,诱导4 h时,重组蛋白的表达量最高(图 3);融合蛋白经Ni-NTA纯化,纯化后的蛋白具有较高的纯度(图 4).
2.1. Tc-duf1943克隆及原核表达载体的构建
2.2. 重组蛋白的诱导表达及纯化
-
卵黄原蛋白是一种高分子质量的磷酸脂糖蛋白(Lipophosphoglycoprotein),在雌激素作用下,可以在除卵巢以外的其他组织中(如昆虫脂肪体、鱼类肝脏、甲壳动物肝胰腺、线虫肠细胞、海胆消化道细胞等)合成,随后分泌进入循环系统,到达卵巢,经卵黄原蛋白受体(Vitellogenin receptor,VgR)介导的内吞作用进入卵母细胞,分解为卵黄脂磷蛋白(Lipovitellin,Lv)、卵黄蛋白(Yolk glycoprotein,YP)和卵黄高磷蛋白(Phosvitin,Pv)等,为正在发育的胚胎提供氨基酸、脂肪、碳水化合物、磷和硫等营养物质[16, 22-25].
重组蛋白表达系统主要有大肠杆菌表达系统、枯草芽孢杆菌表达系统、酵母表达系统、昆虫表达系统、哺乳动物细胞表达系统及植物病毒系统等.其中大肠杆菌表达系统是目前最为成熟的原核表达系统,其对应的表达载体之一pET-28a(+),具有组氨酸融合表达标签,该标签相对分子质量较小,对外源蛋白的空间结构和活性影响较小,能使外源目的蛋白实现融合表达;pET系统的另一个重要优点是在非诱导条件下,外源蛋白处于沉默状态而不表达[26].试验中诱导表达的菌株是E. coli BL21菌株,该菌株缺少lon和ompT基因,是具有T7 RNA聚合酶基因的蛋白酶缺陷株,能减少重组蛋白的降解[27],因此E. coli系统被广阔应用于生物学领域.
卵黄原蛋白功能结构域DUF1943能与微生物的保守组件即病原相关模式分子(Pathogen associated molecular patterns,PAMPs)相结合,发挥免疫活性作用. Li等[28-29]报道Vg的2个功能结构域DUF1943和vWD均能与PAMPs相结合,例如革兰氏阴性菌的脂多糖(Lipopolysaccharide,LPS)、革兰氏阴性/阳性菌的肽聚糖(Peptidoglycan,PGN)、革兰氏阳性菌的脂磷壁酸(Lipoteichoic acid,LTA)和真菌的葡聚糖(Glucan)[30]等结合. Du等[31]发现后生动物大榔头珊瑚Euphyllia ancora,E. ancora的功能结构域Vitellogenin_N,DUF1943和vWD也可作为模式识别受体.而犬弓首蛔虫DUF1943是否具有同样的免疫活性作用,还需后续进一步的试验研究.



 下载:
下载: