-
Ⅱ-Ⅵ族半导体材料ZnS是一种直接带隙半导体材料,不仅因为其出色的物理特性,如能带带隙宽,高折射率,在可见光范围内高透光率,而且其在应用光学、光电子器件等方面的巨大潜力,已经引起了极大关注[1-2].并且ZnS是目前被广泛研究的一种光催化材料,它能在光激发下迅速产生电子-空穴对,并在紫外光照射下具有很高的催化分解有机污染物的活性[3].常见的纳米材料合成方法如湿化学法、气相沉积法等都能用来合成ZnS纳米材料[4].然而这些方法要么合成过程复杂,要么产率低、成本高,不适于ZnS纳米材料大规模合成.
溶胶凝胶自燃烧法是一种将传统溶胶凝胶法和自燃烧法相结合的新型材料合成方法[5].此方法以前驱物中的络合剂为燃料,金属硝酸盐的硝酸根为氧化剂,对干凝胶适当加热后引发自燃烧,在燃烧中形成产物.此方法具有反应时间短、操作步骤简便、原材料廉价、产率高等特点,非常适合纳米粉体材料的大规模合成.目前已广泛应用于多种氧化物纳米材料的合成[6].因此研究利用溶胶凝胶自燃烧法合成ZnS纳米颗粒,有可能提供一种简便高效的硫化物纳米材料合成工艺,具有较强的应用和学术价值.
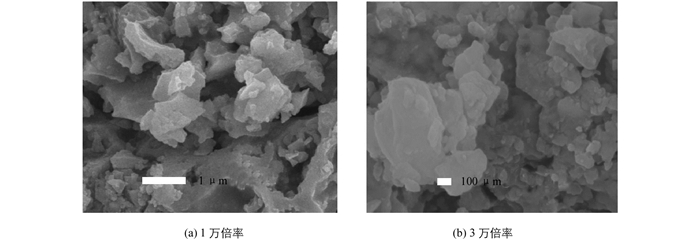
以溶胶凝胶法为基础的ZnS纳米材料合成研究在20年来已有零星报道[7],但是均存在一些缺陷,如:原材料昂贵、操作过程繁杂且产物不稳定等.最近JIANG Y W等[8]利用溶胶凝胶自燃烧法,通过在胶体中引入过量硫脲作为络合剂,在燃烧过程中同时实现nZn/nCd纳米颗粒的还原与硫化,合成了ZnS,CdS和ZnxCd1-xS纳米颗粒.但是重复这一实验发现合成的纳米颗粒粒径均一性很差(图 1).由于ZnS和CdS等半导体纳米材料在光、电、磁和光催化等方面的应用与其晶体结构和颗粒粒径紧密相关,无法得到颗粒粒径均一性较好的纳米颗粒限制了此方法的应用范围.
在JIANG Y W等的研究基础上,通过在前驱物中引入乙二醇和氨基乙酸作为硫脲以外的络合剂,成功利用溶胶凝胶自燃烧法合成了颗粒粒径均一性较好的多晶ZnS纳米颗粒,并且进一步详细研究了前驱物中n(乙二醇)/n(氨基乙酸)、n(氧化剂)/n(燃料)和锌、硫原子摩尔比对样品纳米颗粒粒径分布的影响.研究发现可以通过n(乙二醇)/n(氨基乙酸)来独立优化纳米颗粒粒径的均一性.由于在燃烧法合成中n(氧化剂)/n(燃料)对产物晶体结构、晶化程度和表面性质等多方面都有显著影响[9],而前驱物中锌、硫原子摩尔比会直接影响最终产物中的锌、硫原子摩尔比,会对硫化物光电性能带来影响[10].实现利用n(乙二醇)/n(氨基乙酸)来独立优化纳米颗粒粒径的均一性,有利于基于此合成方法的功能材料设计和性能优化研究.
全文HTML
-
称取定量的硝酸锌(Zn(NO3)2·6H2O,0.003 mol)、硫脲(CH4N2S,0.0045 mol)、乙二醇(C2H6O2,ethylene glycol)和氨基乙酸(C2H5NO2,glycine)(乙二醇与氨基乙酸总摩尔量为0.006 mol,且n(乙二醇)/n(氨基乙酸)=2.8/1),按顺序搅拌溶解在35 mL蒸馏水中.将得到的前驱物溶液放到真空干燥箱中70 ℃下烘干30 h.然后将干凝胶置于水平管式炉的石英管中,用氮气冲洗石英管10 min后关闭气流,并在600 ℃下点燃胶体,燃烧过程持续约1 min.燃烧结束后将样品转移出高温区,通氮气冷却至室温后进行研磨,并在多次清洗后60 ℃下真空干燥.为了进行对比,同时制备了前驱物中只加入硫脲的样品,合成方法与上述一致,只是前驱物络合剂只使用硫脲.
-
使用JSM-7100F型热场发射扫描电子显微镜(SEM)对样品的形貌进行分析,工作电压为10.0 kV,WD为10 mm.制样时将导电胶黏在样品台上,将少量样品均匀涂抹在导电胶上,再进行喷金处理.使用Nano Measurer软件处理样品SEM图像,对每一个样品统计50个纳米颗粒的粒径大小,并计算相应粒径平均值和方差.使用Rigaku D/max-2400X射线衍射仪(XRD)进行物相分析.测试条件:Cu Kα辐射,λ=1.54 nm,工作电压为40 kV,工作电流为60 mA,扫描步进为0.02°.样品的光吸收性能使用岛津UV-2600紫外分光光度计带积分球附件(UV-Vis)进行测量,使用BaSO4作为参考.
1.1. 最佳优化样品制备
1.2. 样品主要测试
-
在JANG Y W等人的研究中前驱物中除了金属硝酸盐只使用了硫脲,硫脲既作为络合剂与Zn离子结合,燃烧热分解时还可以提供形成ZnS所需要的S源[8].虽然文献中没有提供能反映大量纳米颗粒形貌的SEM图片,但我们严格重复此研究工作发现合成得到的产物形貌很差,产物SEM见图 1,其中既有微米尺寸形貌不规则的块状材料,又有少量区域材料表面有纳米颗粒团聚体的堆积.
从基于络合物形成的溶胶凝胶过程分析,硫脲分子中主要起络合作用的原子应该是和C原子双键相连的S原子,而两个—NH2中的N原子络合能力会被削弱,当S原子与金属原子发生配位反应后,两个—NH2中的N原子很难再与其他金属离子发生配位反应[7],只能形成具有较简单网络结构的金属硫脲硫化物(其结构式为(NH2)2—C=S—M—S=C—(NH2)2,M指的是金属离子).所以只使用硫脲做络合剂很难在干凝胶中形成金属离子间的复杂交联网络,也就很难得到尺寸均一的产物[11].为提高胶体网络的复杂性,引入氨基乙酸和乙二醇作为硫脲以外的络合剂.由于不同络合剂中含有官能团的数量和种类不同,所以不同络合剂具有不同的络合能力,常见络合剂官能团的络合能力从高到低排名依次为:—COOH,—OH,—NH2.氨基乙酸中官能团—COOH具有较强的络合能力,与金属离子结合后在原金属硫脲硫化物的基础上,进一步形成具有HO—C=O—M—O=C—OH结构的复杂胶体网络.同时少量的乙二醇的—OH有可能和氨基乙酸的—COOH和—NH2发生缩合反应,将两个氨基乙酸分子连接起来,形成更复杂的胶体网络.此外,乙二醇在干凝胶中还能起到分散剂作用,其简单的分子结构与较小的分子量使其容易均匀分散在各个络合物之间,并且乙二醇与络合物分子团之间结合较弱,在高温燃烧中容易迅速断开加速胶体解体,有利于硫化反应的均匀发生.因此在此研究中,在前驱物中除了硫脲还加入适量的乙二醇和氨基乙酸作为络合剂,并通过对前驱物组分进行优化,最终得到尺寸均一性较好的ZnS纳米颗粒.
图 2是样品的X射线衍射图谱,其中在28.54°,47.48°和56.30°的较强衍射峰分别对应于闪锌矿ZnS(JCPDS号:77-2100)的(111)、(220)和(311)晶面,说明合成得到的ZnS纳米颗粒主要为闪锌矿结构.在27°附近较为微弱的衍射峰对应于纤锌矿ZnS(JCPDS号:79-2204)的(100)晶面,表明在合成过程中有少量的闪锌矿ZnS转变为纤锌矿ZnS.通过测量闪锌矿ZnS(220)晶面对应衍射峰的半高宽(FWHM),并用Scherrer公式可以估算出样品对应的平均晶粒尺寸为7.2 nm.
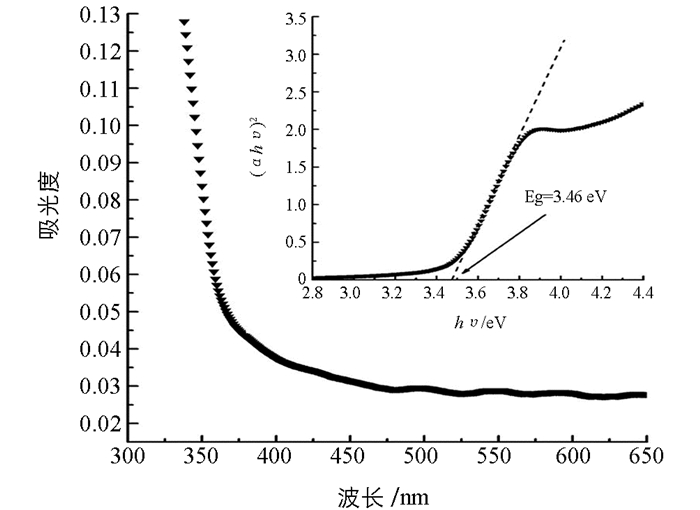
图 3为样品的紫外-可见吸收光谱,可以看到陡峭光滑的吸收边出现在360 nm附近,说明样品具有良好的紫外光吸收性能,然后在400 nm到650 nm的范围内也表现出一定的吸收强度.考虑到燃烧反应激烈以及多晶纳米材料表面稳定性较差,我们推测这一大范围吸收肩应该是来源于材料的表面缺陷能级和表面态能级[12].基于紫外-可见光吸收光谱,利用Tauc公式可以计算样品的能带带隙大小[13].从图 3插图中可以读出ZnS的能带带隙为3.46 eV,这一结果与先前报道的ZnS带隙数值吻合.
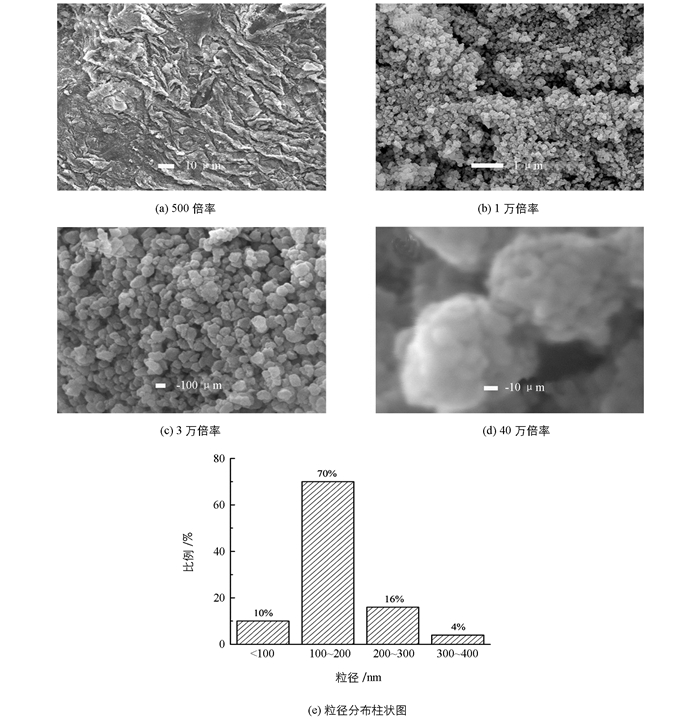
图 4为样品的SEM图像. 图 4(a)显示在500倍率下大量样品均匀涂抹在导电胶表面,表面光滑平整没有观察到大块的胶体残骸. 图 4(b)显示1万倍率下,样品是由大量尺寸均一形状相对规则的纳米颗粒堆叠起来组成的,并且从表面的裂缝中可以观察到内部也是纳米颗粒,说明样品是完全由纳米颗粒堆叠而成的,而不是少数纳米颗粒堆积在大块材料表面.放大倍率更高的图 4(c)为3万倍率下图像,显示出纳米颗粒的粒径大多在160 nm左右,与之前只加入硫脲的样品相比很明显纳米颗粒尺寸更为均一.当在放大倍率40万倍下观察,发现在3万倍率下观察到的纳米颗粒其实是由直径在10 nm左右的颗粒生长在一起组成的(图 4(d)),这些小颗粒的粒径与XRD分析中得到的晶粒大小符合,说明合成得到的ZnS纳米颗粒是多晶纳米颗粒.从图 4(e)的粒径分布柱状图中可以看到,纳米颗粒粒径小于100 nm的占10%,粒径处于100~200 nm的占绝大数为70%,粒径大小为200~300 nm的占到16%,仅有4%的纳米颗粒粒径在300~400 nm之间,这说明样品纳米颗粒的粒径具有良好的均一性.
以上表征说明,通过引入适量的乙二醇和氨基乙酸成功利用溶胶凝胶自燃烧法合成了颗粒尺寸均一性较好的ZnS纳米颗粒.在这基础上,希望可以进一步阐明前驱物组分对样品纳米颗粒粒径大小和均一性的影响,进而提供一种基于自燃烧法的可靠ZnS纳米颗粒合成工艺.下面通过对前驱物中以下3种组分比值的控制对比实验来研究乙二醇和氨基乙酸引入后前驱物组分对纳米颗粒粒径分布的影响.
首先,n(氧化剂)/n(燃料)是溶胶凝胶自燃烧法中最重要的实验影响因素,它直接决定了燃烧温度和燃烧速度,进而对产物成相、晶化、颗粒尺寸和表面性质产生重要影响.为了表述方便,在后续讨论中前驱物中的n(氧化剂)/n(燃料)用nZn/nC来代表.其次,在以络合物交联网络形成为基础的溶胶凝胶过程中,当络合剂为有机酸时加入乙二醇能显著提高胶体内部网络的复杂性和稳定性,两者之间的比值对产物会产生显著的影响,前驱物中合适的n(乙二醇)/n(有机酸),有利于获得均一性更好的产物[14].而相比其他有机酸络合剂,氨基乙酸具有更高的燃烧活性并能带来更好的胶体均一性,更适合惰性气氛下的燃烧反应[15].最后,在ZnS制备的燃烧反应过程中,必然会伴随含S气体分子的产生(如:H2S和SO2),带来最终产物中S原子的损耗.因此要得到ZnS,前驱物中应加入过量的S.但在控制nZn/nC的情况下,nZn/nS的调节同时还会影响乙二醇和氨基乙酸的总量,从而对样品纳米颗粒的粒径分布产生一定影响.
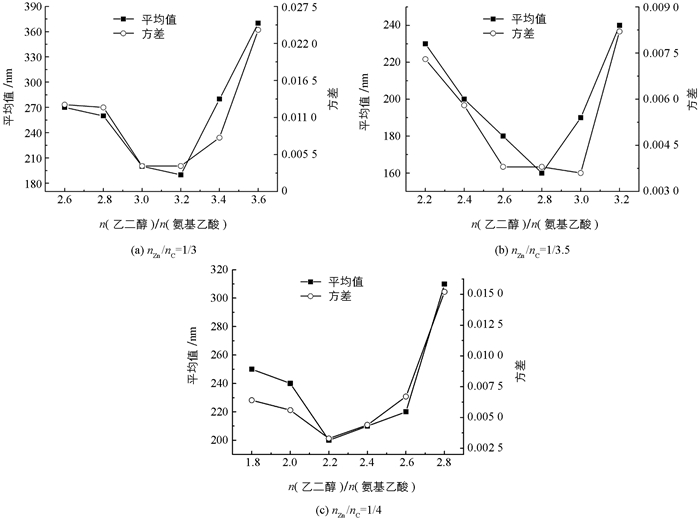
在对比实验1中保持nZn/nS在1/1.5,研究nZn/nC=1/3、1/3.5和1/4的情况下n(乙二醇)/n(氨基乙酸)对ZnS纳米颗粒粒径分布的影响.用纳米颗粒的平均粒径和方差来表征样品粒径分布(图 5).可以看到随着n(乙二醇)/n(氨基乙酸)增加,三组样品对应的平均粒径和方差都是先减小后增大,均存在最小值.在nZn/nC=1/3和1/4两组中,平均粒径和方差的最小值在同一样品上取得,代表此样品粒径最小且均一性最好,此样品对应的n(乙二醇)/n(氨基乙酸)即为最佳比例.而在nZn/nC=1/3.5组中,n(乙二醇)/n(氨基乙酸)=2.8/1时具有最小平均粒径160 nm,相应方差为0.003 8,而当n(乙二醇)/n(氨基乙酸)=3.0/1时取得最小方差0.003 6,相应平均粒径190 nm.综合两样品的方差和平均粒径差异,取n(乙二醇)/n(氨基乙酸)=2.8/1为nZn/nC=1/3.5时的最佳比例.
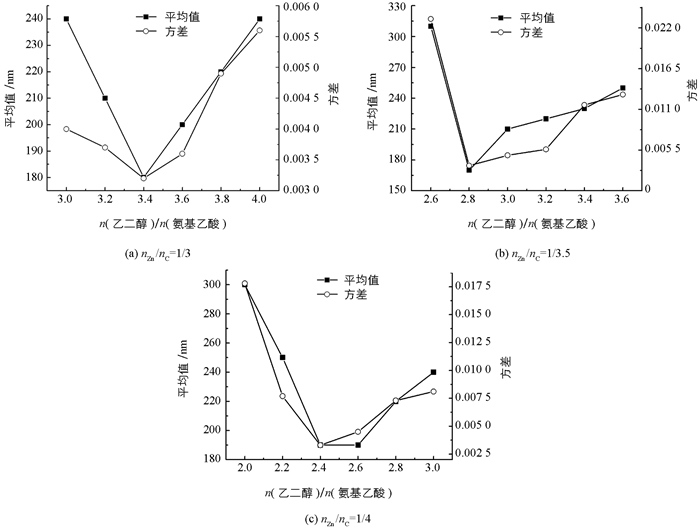
在对比实验2中改变nZn/nS为1/2,同样研究了nZn/nC的比例为1/3、1/3.5和1/4的情况(图 6).整体来看与nZn/nS=1/1.5时保持相同的变化趋势,粒径平均值和方差均随n(乙二醇)/n(氨基乙酸)的增加而先减小后增大,类似对比实验1在不同nZn/nC下均能找到最佳的n(乙二醇)/n(氨基乙酸).
将对比实验1和2中6个最佳n(乙二醇)/n(氨基乙酸)对应样品的粒径分布结果列在表 1中进行比较.首先同一对比实验中不同nZn/nC下最佳的n(乙二醇)/n(氨基乙酸)对应样品的方差差距很小且数值也很小,说明通过调节n(乙二醇)/n(氨基乙酸)在不同n(氧化剂)/n(燃料)都能得到颗粒尺寸均一性良好的样品.但是各组平均粒径大小受到对应的n(氧化剂)/n(燃料)的影响,在nZn/nC=1/3.5时具有最小的平均粒径160 nm,这一样品也就是此研究中的最佳优化样品.其次发现每组最佳的n(乙二醇)/n(氨基乙酸)是随n(氧化剂)/n(燃料)的下降而逐步下降的,这一规律可以帮助在新的n(氧化剂)/n(燃料)下快速找到合适的n(乙二醇)/n(氨基乙酸).最后发现在nZn/nS不同的两对比实验中上述两条结论均保持不变.这一系列对比实验说明我们可以通过调控n(乙二醇)/n(氨基乙酸)来独立优化样品纳米颗粒的粒径均一性.由于在燃烧法合成中n(氧化剂)/n(燃料)对产物晶体结构、晶化程度和表面性质等多方面都有显著影响,而nZn/nS能对ZnS纳米颗粒的光电性能进行调控,能通过调控n(乙二醇)/n(氨基乙酸)来独立优化样品纳米颗粒的粒径均一性,有利于基于此合成方法的功能材料设计和性能优化研究.
-
本研究中成功利用溶胶凝胶自燃烧法合成了具有闪锌矿结构的ZnS纳米颗粒,合成的ZnS纳米颗粒粒径均一性较好(平均尺寸为160 nm)且形状较为整齐,具有良好的光吸收性能,其能带带隙为3.46 eV.并通过对前驱物中n(氧化物)/n(燃料)、n(乙二醇)/n(氨基乙酸)、n(硝酸锌)/n(硫脲)等因素的调节,系统地研究了各因素对ZnS纳米颗粒形貌的具体影响.研究发现在不同n(氧化剂)/n(燃料)下通过调节n(乙二醇)/n(氨基乙酸)均能获得形貌均一的ZnS纳米颗粒,并且最佳的n(乙二醇)/n(氨基乙酸)随n(氧化剂)/n(燃料)的下降而逐步下降;在不同n(氧化剂)/n(燃料)下,形貌最佳的样品的纳米颗粒的粒径大小会有所不同,并且特定比例下存在最小值;上述两条结论在不同nZn/nS比例下均成立.这一研究成果不仅提供了一种简单高效的ZnS纳米颗粒合成工艺,并且实现了利用n(乙二醇)/n(氨基乙酸)独立优化纳米颗粒粒径均一性,有利于基于此合成方法的功能材料设计和性能优化研究,同时也为溶胶凝胶自燃烧法在硫化物纳米材料合成中的进一步应用提供了理论和实验基础.



 下载:
下载: