-
土壤的自然成土过程是一个缓慢的酸化过程[1].受土壤发育程度的影响,酸性土壤主要分布在热带和亚热带地区.近年来人类活动所导致的气候和土地利用方式变化大大加速了土壤酸化的进程[2-3].严重的土壤酸化引发一系列的生态环境和农业生产问题[4].酸沉降[5-6]和氮肥过量施用是人类活动加速土壤酸化的主要原因[7-8].土壤酸化导致盐基养分离子损失,重金属离子被活化和对植物产生铝毒害等系列问题[9-13].土壤中氢离子和铝离子数量增加的过程即是土壤酸化的过程.土壤的交换性酸含量是评价土壤酸化程度的主要指标[11].其测定原理是用含某种阳离子的盐溶液作为交换剂将土壤胶体上所吸附的H+和Al3+交换下来进行测量.常用的交换剂有非pH值缓冲体系盐溶液(KCl,NaCl,BaCl2,MgCl2等)和具有一定pH值缓冲能力的盐溶液(NaAc,KAc,CuAc2等)[14].也可将中性盐和有机弱碱配合使用作为提取剂,如BaCl2-三乙醇胺(TEA)[15].目前国内外应用最广泛的是采用KCl淋溶法测定土壤交换性酸含量[16-17].由于K+的交换能力较弱,KCl淋溶法测得的土壤交换性酸含量较低.杨剑虹等[15]建议采用KCl淋溶法测量交换性酸应乘以校正系数1.3~1.7,但已发表的研究论文中大多并未进行校正.
有研究者采用BaCl2-TEA作为交换剂来测量土壤交换性酸含量[18].该方法是利用Ba2+的强交换能力将土壤吸附的H+,Al3+交换出来,而交换出来的H+,Al3+被有机碱TEA及时中和,使交换反应快速而完全. BaCl2-TEA交换法无需连续淋洗和多次交换,只需一次平衡交换即可,操作更为简洁.但该方法的测定原理是基于测定加入的和剩余的TEA含量之差来计算出土壤交换性酸含量,即所消耗的TEA含量必须全部用于与H+和Al3+水解产生的H+反应.但在实际情况下,土壤中的组成成分也会与有机碱TEA发生中和反应,从而消耗TEA,造成土壤交换性酸测定结果偏高,但较少发现相关的研究报道.本研究拟探讨BaCl2-TEA提取法和KCl淋溶法在不同有机质质量分数酸性土壤上的交换性酸测定结果,最终揭示土壤有机质质量分数对交换性酸测定结果的影响.
全文HTML
-
供试土样为重庆市丰都县酸化改良项目土样,土壤为施入不同含量有机肥进行培育改良的酸化紫色土和黄壤,于2017年采集.因此,所得土样为一系列不同有机质质量分数的酸化土壤.所有土样自然风干后过筛备用.
-
土样基本理化性质采用常规分析方法[19]. pH值测定采用电位法(土水比为1: 2.5);有机质采用重铬酸钾容量法—外加热法;全氮采用半微量凯氏定氮法;碱解氮采用碱解扩散法;全磷采用NaOH熔融—钼锑抗比色法;有效磷采用HCl-NH4F提取—钼锑抗比色法;全钾采用NaOH熔融—火焰光度法;速效钾采用NH4Ac提取—火焰光度法;交换性K+、交换性Na+采用NH4Ac交换—火焰光度法;交换性Ca2+、交换性Mg2+采用NH4Ac交换—原子吸收分光光度法(Z-5000,日本日立).
-
KCl淋溶法交换性酸的计算公式为:
式中:V1为样品滴定中NaOH消耗量(mL);V0为空白滴定中NaOH消耗量(mL);c为NaOH标准溶液的浓度(mol/L);m为土样干质量.
BaCl2-TEA提取法交换性酸的计算公式为:
式中:V1为样品滴定中HCl消耗量(mL);V0为空白滴定中HCl消耗量(mL);c为HCl标准溶液的浓度(mol/L);m为土样干质量.
交换性盐基总量=交换性K++交换性Na++交换性Ca2++交换性Mg2+;
有效阳离子交换量(ECEC)=交换性酸+交换性盐基总量;
盐基饱和度(%)=交换性盐基/ECEC.
利用Excel软件和SPSS 13.0软件对数据进行统计分析.
1.1. 土壤样品准备
1.2. 交换性酸测定方法
1.3. 基本理化性质分析
1.4. 数据处理
-
在仅考虑实验操作过程的情况下,两种土壤交换性酸测定方法各有难易程度和优缺点.在实验试剂配制方面,KCl淋溶法采用的是1 mol/LKCl作为提取剂,配制方法简单.而BaCl2-TEA提取法为pH=8.0的0.25 mol/LBaCl2和0.055 mol/L TEA溶液,由于TEA为特别黏稠的液体试剂,准确量取体积较为困难,此外还需要用TEA或低浓度HCl将溶液pH值调至8.0,所以BaCl2-TEA提取法的提取剂配制更为复杂.而在滴定剂方面,KCl淋溶法采用标准碱溶液,由于标准碱溶液的浓度受空气中CO2影响较大而易发生变化,因此标准碱溶液不能长期保存且每次使用前均需进行浓度标定. BaCl2-TEA提取法采用标准酸溶液作为滴定剂,其性质稳定,浓度不易发生改变,可长期保存使用且无需再次标定. KCl淋溶法采用的是多次连续淋洗后,滤液还需经高温煮沸排除CO2后才能进行滴定,而BaCl2-TEA提取法为一次平衡后直接过滤滴定.但在实验结束后,BaCl2-TEA提取法中所有与Ba2+接触过的器皿需用酸洗液浸泡过后再进行进一步清洗,这是由于Ba2+会与CO32-等阴离子生成白色沉淀附着在器皿表面,造成器皿的污染.总的来说,BaCl2-TEA提取法测得土壤交换性酸含量的操作更为简洁,但该方法无法区分交换性酸中的交换性H+和Al3+含量.
-
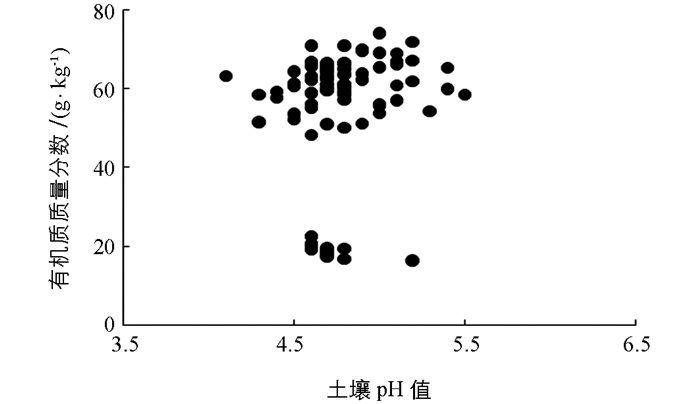
供试土壤的基本理化性质如表 1和表 2所示,土壤pH值变化范围为4.1~5.5,变幅较小,但均处于强酸化状态.土壤有机质质量分数均较高,且变幅较大,从最低的16.3g/kg至最高的73.9g/kg.变幅较大的有机质质量分数有助于明确不同土壤有机质质量分数对土壤交换性酸测得结果的影响.但唯一不足的是样品的有机质质量分数分布不均,大多集中在50~70 g/kg间(图 1).各土壤速效养分含量均较为丰富,碱解氮、有效磷、速效钾质量分数分别为(301±82),(56.5±23.9)和(268±112)mg/kg.土壤全氮、全磷、全钾质量分数为(3.28±0.92),(1.29±0.52)和(19.2±1.57) g/kg.土壤交换性盐基离子含量均值从大到小依次为:交换性Ca2+(4.44±3.19 cmol(+)/kg)、交换性Mg2+(1.52±0.96 cmol(+)/kg)、交换性K+(0.76±0.28 cmol(+)/kg)、交换性Na+(0.14±0.12 cmol(+)/kg)(表 2).采用BaCl2-TEA提取法测得的土壤交换性酸含量为27.7±7.35 cmol(+)/kg,而采用KCl淋溶法测得的土壤交换性酸含量为3.26±1.22 cmol(+)/kg,可以看出二者的测定结果均值相差达8倍以上,因此有必要探讨两种土壤交换性酸测定方法在高有机质质量分数土壤中的适用性.由两种交换性酸计算得到的土壤ECEC值分别为34.6±4.84cmol(+)/kg(BaCl2-TEA提取法)和8.54±3.66 cmol(+)/kg(KCl淋溶法).进一步计算得到的盐基饱和度也相差较大,由BaCl2-TEA提取法测得交换性酸计算得到的盐基饱和度为21.3±15.7%,由KCl淋溶法测得交换性酸计算得到的盐基饱和度为75.8±13.6%.
-
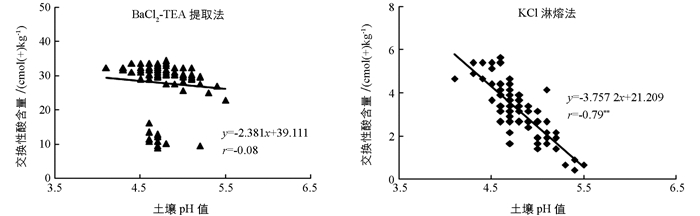
本研究中,BaCl2-TEA提取法测得的土壤交换性酸含量远大于KCl淋溶法测得的土壤交换性酸含量(图 2). BaCl2-TEA提取法中加入了高容量的有机弱碱TEA,Ba2+从土壤胶体上交换下来的致酸离子会被TEA及时中和,从而使交换反应不断进行.而KCl淋溶法是利用多次连续淋溶以促进交换反应的正向进行.理论上无论采取何种方法测定土壤交换性酸含量,其测定结果应差异不大.但BaCl2-TEA提取法采用二价的Ba2+去交换土壤胶体所吸附的致酸离子,而KCl淋溶法是以一价的K+作为交换阳离子,尽管所采用Ba2+的浓度低于K+,但Ba2+对H+和Al3+的交换能力显著高于K+.因此,可能导致BaCl2-TEA提取法测得的土壤交换性酸含量大于KCl淋溶法.此外,土壤的有机质质量分数等其他因素也可能是导致BaCl2-TEA提取法测定结果大于KCl淋溶法的原因.
土壤交换性酸含量是反映土壤酸化程度的一个容量指标,而土壤pH值是反映土壤酸化程度的一个活性指标.通常土壤pH值越低,土壤的交换性酸含量越高.将所测得的土壤pH值与两种方法测得的土壤交换性酸含量进行相关性分析可以发现(图 2),BaCl2-TEA提取法测得土壤交换性含量与土壤pH值的相关系为-0.08,而KCl淋溶法测得土壤交换性酸含量与土壤pH值的相关系数为-0.79,达到了极有统计学意义水平(p<0.01).所以从与pH值相关性的角度考虑,采用KCl淋溶法测得的土壤交换性酸含量更为合理,而采用BaCl2-TEA提取法测得土壤交换性含量存在较大误差.
-
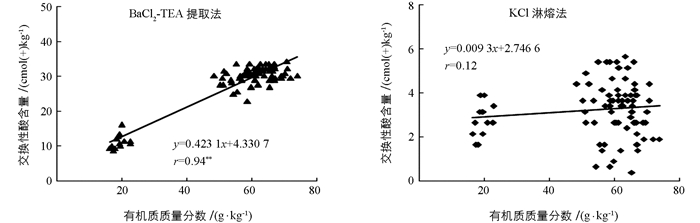
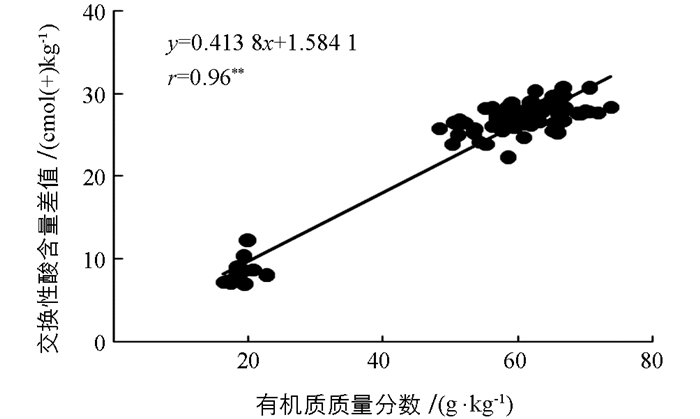
通过pH值与土壤交换性酸含量进行相关性分析后发现BaCl2-TEA提取法测得的土壤交换性酸含量误差更大.进一步将土壤有机质与土壤交换性酸进行相关性分析后可以发现BaCl2-TEA提取法测得的土壤交换性酸含量与土壤有机质间存在极有统计学意义的正相关性(r=0.94**),而KCl淋溶法测得的土壤交换性酸含量与有机质间的相关性无统计学意义(r=0.12)(图 3).所以,土壤有机质对BaCl2-TEA提取法测得的土壤交换性酸含量影响较大,而对KCl淋溶法影响较小.土壤有机质的主要成分是腐殖酸,而腐殖酸分子中含有各种功能基团,包括芳香族和脂肪族化合物上的羧基(R-COOH)和酚羟基(酚-OH)[20].这些功能基团均会与TEA发生反应而消耗TEA,造成计算得到的土壤交换性酸结果偏高,产生误差.土壤有机质质量分数越高,误差越大.所以,BaCl2-TEA提取法测得交换性酸与土壤pH值相关性无统计学意义,反而与土壤有机质质量分数呈极有统计学意义的正相关关系,也恰好说明了土壤有机质存在对该方法产生影响,造成测量结果偏高.进一步将BaCl2-TEA提取法与KCl淋溶法测得的土壤交换性酸含量差值与土壤有机质质量分数进行相关性分析可以发现二者的正相关性极有统计学意义(r=0.96**)(图 4).这说明了随着土壤有机质质量分数的增加,两种方法所测得的结果相差越大.而在低有机质质量分数的土壤中,两种方法的测得结果才会彼此接近.因此不宜采用BaCl2-TEA提取法测高有机质质量分数酸性土壤的交换性酸含量.
-
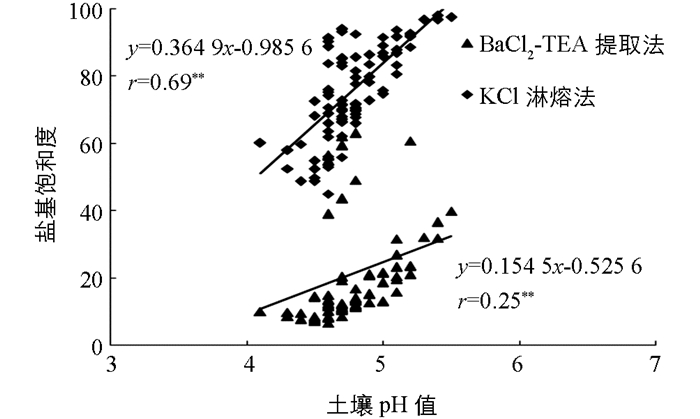
未酸化的土壤处于盐基饱和状态,土壤胶体上的负电荷位点全部被盐基离子(K+,Na+,Ca2+,Mg2+)所占有.当土壤酸化后,盐基离子被致酸离子H+和Al3+所取代,造成土壤盐基不饱和.所以,土壤酸化越严重,盐基饱和度越低[21].将土壤pH值与两种交换性酸测定方法计算得到的盐基饱和度进行相关性分析(图 5).由KCl淋溶法测得的土壤交换性酸计算得到的盐基饱和度与pH值相关系数为0.69,达到极有统计学意义水平.而由BaCl2-TEA提取法测得的土壤交换性酸计算得到的盐基饱和度与pH值相关系数为0.25,有统计学意义. KCl淋溶法测得的土壤交换性酸计算得到的盐基饱和度与pH值的相关性更好,说明由该方法计算得到的盐基饱和度可更好地表征土壤的酸化程度.所以采用KCl淋溶法测定土壤交换性酸含量更为合理.
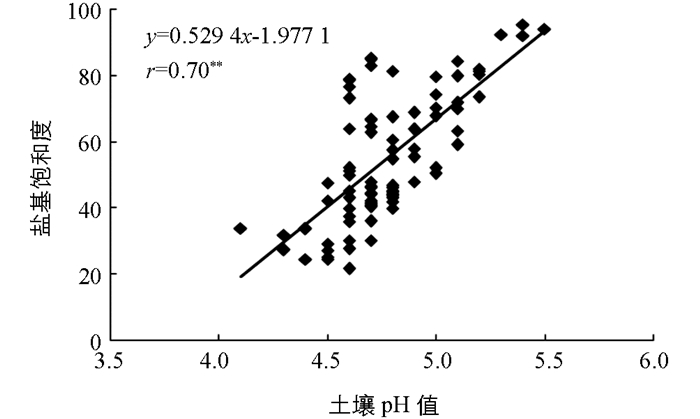
通常认为当土壤pH值<5.0时,土壤盐基饱和度<30%;当土壤pH值在5.0~5.5之间时,土壤盐基饱和度在30%~60%之间;当土壤pH值在5.5~6.0之间时,土壤盐基饱和度在60%~80%之间;当土壤pH值在6.0~7.0之间时,土壤盐基饱和度在80%~100%之间[22].本研究中所用土样pH值在4.8~5.5之间.从表 2和图 5可以看出,受土壤有机质质量分数的影响BaCl2-TEA提取法计算得到的盐基饱和度偏低.而KCl淋溶法计算得到的盐基饱和度偏高,这可能是由于K+的交换能力较弱,测得的土壤交换性酸含量偏低造成的.按照杨剑虹等[15]的建议,对KCl淋溶法测得得土壤交换性酸乘以校正系数1.5,此时土壤的交换性酸含量从测得的3.26±1.22 cmol(+)/kg增加至4.89±1.84cmol(+)/kg.由此进一步计算得到的土壤盐基饱和度从75.8±13.6%下降至55.2±19.4%.将乘以校正系数后的盐基饱和度与土壤pH值进行相关性分析(图 6),二者的相关性水平的正相关性仍极有统计学意义(r=0.70**),而此时的土壤pH值与其对应的盐基饱和度才与通常的认识更加吻合.
值得注意的是,理论上土壤交换性酸是指的土壤胶体所吸附的H+和Al3+,而在实际操作中土壤交换性酸的测量属于操作形态上的定义.各种测定方法理论上都能将土壤胶体所吸附的H+和Al3+交换下来进行测得,但实际上受土壤性质和反应条件等因素的影响,真实的交换效果并不知晓.因此,尚无标准方法和标准样品来对各种土壤交换性酸测定方法的准确性进行比较和验证.从通常理解的盐基饱和度和土壤pH值的对应范围来说[22],KCl淋溶法测得土壤交换性酸含量偏低,需乘以1.5左右的系数进行校正.若仅从盐基饱和度与土壤pH值相关性的角度出发,KCl淋溶法测定土壤交换性酸更为合理,而BaCl2-TEA提取法受土壤有机质质量分数等因素的影响而存在较大的误差.
2.1. 两种方法在操作上的优劣性
2.2. 土壤基本理化性质
2.3. 土壤交换性酸与土壤pH值相关性分析
2.4. 土壤交换性酸与土壤有机质相关性分析
2.5. 土壤盐基饱和度含量分析
-
1) 土壤有机质中的腐殖酸会与BaCl2-TEA提取法中的TEA发生反应,从而使计算得到的土壤交换性酸的结果偏高,产生误差.因此,不宜采用BaCl2-TEA提取法测定高有机质质量分数酸性土壤的交换性酸含量.
2) 采用KCl淋溶法测得的土壤交换性酸含量及由其计算得到的盐基饱和度与土壤pH值的相关性均极有统计学意义,因此可选用KCl淋溶法作为土壤交换性酸的测定方法.但该方法中由于K+对Al3+的交换能力较弱,造成测的的土壤交换性酸含量偏低.因此,可对KCl淋溶法测得的土壤交换性酸含量乘以1.5左右的校正系数,以便能真实地反映出土壤交换性酸含量.



 下载:
下载: