-
陆生植物进化时期,大气CO2浓度发生了剧烈变化.在上新世晚期(the Late Pliocene),大气中的CO2浓度为150 μmol/mol,是有记载以来的最低浓度,在18 000~20 000年前的末次冰河时期(LGM),大气中CO2的浓度保持在180~190 μmol/mol之间;到工业革命前,CO2浓度升高到了270 μmol/mol[1].随着工业活动的增加、化石燃料的燃烧和森林的大量砍伐,最近200年CO2浓度急剧上升,目前大约为400 μmol/mol,并预计还将继续上升,在2 100年可能达到700 μmol/mol[2-4].
由于大气CO2浓度是植物光合作用底物,CO2浓度的急剧变化必然对植物的生长、发育和繁殖产生重大影响,从而影响植物生产力,决定群落结构和陆地生态系统功能[2, 5].由于近年来CO2浓度急剧升高,大量的研究关注了大气CO2浓度升高是否或者如何影响了植物、群落及生态系统生产力[6-13].地质历史时期的低CO2浓度,对植物进化产生重要影响,其中LGM时期被认为是限制植物生长和生理功能的一个重要时期[14-16].Cunniff等[17]研究表明,LGM时期谷物进化受到了低CO2浓度的极大限制.现代C3植物在模拟LGM时期的低CO2浓度下生长,光合和生物量生产下降40%~70%[2, 18],存活率下降20%~30%[19],甚至导致不能繁殖[15].基于树木年轮,通过碳同位素技术比较研究美国加州南部拉布雷亚沥青坑(La Brea tar pits)冰期孑遗和现代刺柏Juniperus胞间CO2浓度(Ci)及胞间CO2浓度和大气CO2浓度比例(Ci/Ca)得出直接证据:冰河时期植物生理特征受到低CO2浓度的限制显著[20].
尽管地质史上低CO2浓度对植物个体[15, 18-19, 21]、群落种类组成[21-23]、植被分布[24]及生态系统功能[25-28]等也产生了重要影响,甚至影响了早期人类文明和农业的起源[29],但目前对低CO2浓度的影响研究还比较少.Ward等[3]研究了在末次冰河时期低CO2浓度对C3和C4植物生理和生长的影响;Gergart等[1]对末次冰河时期低CO2浓度对植物的影响进行了全面综述,包括研究方法,低浓度对植物个体生物量产生、分配、生殖和生存的影响等.Sage等[2]研究了植物对过去CO2浓度的响应,并指出低CO2浓度可能是植物进化的“过滤器”(filter).有极少研究关注了植物对冰河时期、前工业革命时期、当前和将来整个CO2浓度连续变化的响应,发现当CO2浓度从冰河时期升高到目前浓度时,其生长的增加幅度远远大于从目前浓度升高到本世纪末可预测的浓度[30-31].研究者通过meta分析,也发现高CO2浓度(>500 μmol/mol)确实增加了植物的光合作用和生物量.然而,增加幅度仅为10%~20%,远远低于预测的50%左右[32-34].因此,冰河时期CO2浓度的微小改变可能会对植物的生物量产生很大影响[1],而在“未来”时间尺度上高CO2浓度的改变对植物的影响可能会下降.
本研究提出以下假设:现代植物在过去低CO2浓度下,其生长将受到极大限制,这种限制却为适应将来高CO2浓度提供可能,也即植物对过去低CO2浓度的极度适应将限制其对将来高CO2浓度的响应.由于一年生草本植物生长速率较快,对CO2浓度等环境因子变化十分敏感,能够快速而客观地表达植物与外部环境的相互关系[35],本研究选择6种常见草本植物,通过模拟“过去、现在和未来”CO2浓度,探讨其生物量积累和分配对不同CO2浓度的响应,研究植物对过去CO2浓度的响应和对将来CO2浓度的响应之间的关系.
HTML
-
实验在荷兰乌特勒支大学特制的步入式CO2浓度控制生长室内完成.以6种常见的一年生草本植物泥胡菜Hemistepta lyrata、网果酸模Rumex chalepensis、野豌豆Vicia Sepium、藜Chenopodium album、风轮菜Clinopodium chinense和玉米石Sedum album为研究材料.所有种子收集于2011年中国重庆地区.2012年3月15日在当前CO2浓度(400 μmol/mol)生长室下进行发芽,出苗后每个物种选取长势基本一致的32株幼苗分成3组移栽于0.4 L内盛等量沙子的花盆中,置于3个不同的CO2生长室(Reftech,Sassenheim,The Netherlands;见http://www.reftech.nl/):3个CO2生长室内CO2浓度分别设定为150 μmol/mol(代表过去冰河时期的低CO2浓度,实测浓度在150~180 μmol/mol间波动)、400 μmol/mol(代表当前CO2浓度,实测浓度在400~420 μmol/mol间波动)和700 μmol/mol(代表可预测的将来高CO2浓度,实测浓度在700~750 μmol/mol间波动).CO2浓度由红外气体分析仪连续跟踪测定.低CO2浓度生长室内的低CO2浓度由分子筛滤技术控制,筛滤器由CMC Instruments GmbH制造(type PG 1500 L,Eschborn,Germany).高CO2浓度由气罐往生长室内添加控制.为了不影响生长室内的CO2浓度水平,进入生长室内的研究人员必须佩戴自制的CO2收集器.每个生长室设置光照条件为450 μmol/(m2·s),光期为10 h,温度为25 ℃/18 ℃,相对湿度为70%.温度、相对湿度和光照强度等环境因子均采取电子自动监控,以保证所有生长室内的植物除了CO2浓度外其他环境因子均保持一致.每个物种6个处理,每个处理8个重复.每2 d对幼苗补充一次稀释2倍的Hoagland营养液,待幼苗第一片真叶完全展开后,对每个物种中的8盆进行初始收获.初始收获后其他的幼苗仍然保持水肥充足.
实验处理时间为3周.3周后分成地上部分和地下部分进行收获,分别称量鲜质量后装入牛皮纸袋,置入烘箱中105 ℃杀青15 min,然后在80 ℃下烘干至恒质量,再称量各部分干质量,进一步计算总生物量(total biomass),根质量分数(Root mass fraction,RMF)=根生物量/总生物量;干物质质量分数(Dry matter content,DMC)=总干质量/总鲜质量;叶干物质质量分数(Leaf dry matter content,LDMC)=总叶干质量/总鲜质量;相对生长速率(Relative growth rate,RGR)=(lnW2-lnW1)/(T2-T1)(W1为第一次取样时(T1)的植物干质量;W2为第二次取样时(T2)的植物干质量).
由于实验结束时,在低CO2浓度生长室内的8株风轮菜全部死亡,玉米石死亡5株,存活的3株植物也极小,因而这2个物种在低CO2浓度下的数据无法进行数据统计分析.本研究只针对其他4个物种,利用SPSS20.0进行不同CO2浓度和不同物种间双因素显著性分析,同时将所有参数对不同CO2浓度升高阶段的响应幅度进行独立样本t检验.
-
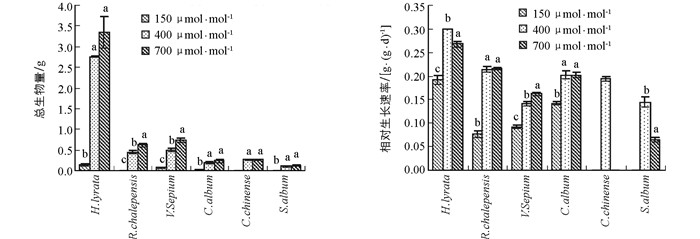
6种草本植物的生物量均随CO2浓度升高呈现出增加的趋势(图 1).网果酸模和野豌豆随CO2浓度升高,生物量显著性增加;泥湖菜和藜和玉米石在CO2浓度升高过程中,其生物量都有所增加,但其生物量在CO2浓度从当前400 μmol/mol升高到未来700 μmol/mol的过程中未达到显著水平.当CO2浓度从过去150 μmol/mol升高到当前400 μmol/mol时,泥胡菜、网果酸模、野豌豆和藜的增加幅度分别为94.9%,97.7%,86.3%和90.1%;但当CO2浓度从当前400 μmol/mol升高到将来可预测的700 μmol/mol时,其增加幅度降低为17.6%,27.9%,33.0%和21.6%,且2组响应幅度之间差异具有统计学意义(表 1).可见,CO2浓度从过去低CO2浓度升高到目前CO2浓度时的刺激作用远大于从目前到将来CO2浓度.双因素方差表明,生物量在物种和CO2浓度处理间存在显著的交互作用(表 2).
-
野豌豆的相对生长速率随CO2浓度升高呈显著增加的趋势,网果酸模的相对生长速率随CO2浓度从过去150 μmol/mol升高到当前400 μ mol/mol阶段呈显著性增加趋势,且2物种CO2浓度从过去150 μmol/mol升高到当前400 μmol/mol时增加幅度显著大于CO2浓度从现在400 μmol/mol升高到将来700 μmol/mol;泥湖菜和藜的相对生长速率在CO2浓度从过去150 μmol/mol升高到当前400 μmol/mol时显著增加,但当CO2浓度从现在400 μmol/mol升高到将来700 μmol/mol时,出现了下降趋势;玉米石的相对生长速率在CO2浓度从现在400 μmol/mol升高到将来700 μmol/mol时也显著下降(图 1,表 1).方差分析表明,相对生长速率在不同物种间差异具有统计学意义,且在不同物种和CO2浓度处理间存在显著的交互作用(表 2).
-
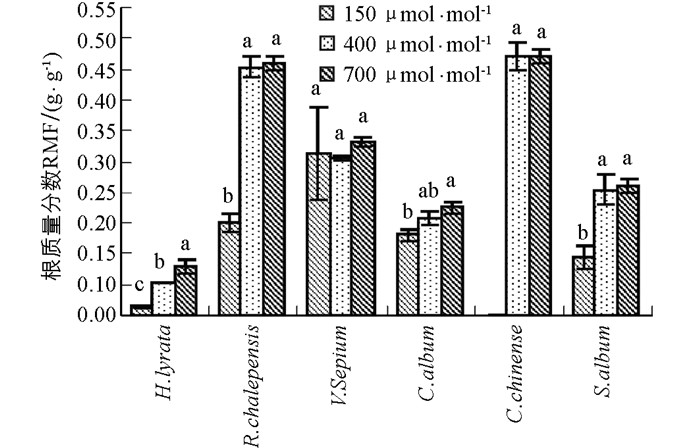
6种草本植物的根质量分数均随CO2浓度升高呈现出增加的趋势(图 2).除了野豌豆,泥胡菜、网果酸模、藜和玉米石这4种植物对过去CO2浓度升高的响应幅度显著大于对将来CO2浓度的升高响应幅度(图 2,表 1).野豌豆在CO2浓度从150 μmol/mol升高到700 μmol/mol时,其根质量分数仍然出现了升高的趋势(图 2,表 1).同样,根质量分数在不同物种间差异具有统计学意义,且在不同物种和CO2浓度处理间存在显著的交互作用(表 2).
-
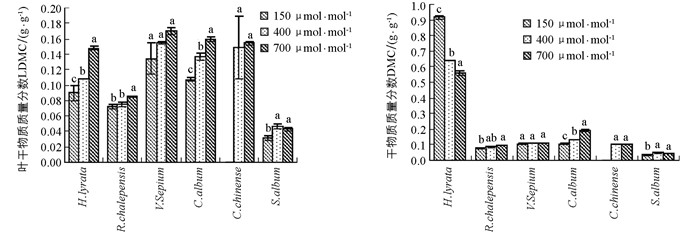
除了玉米石,CO2浓度升高总体上促进了其他5种植物叶干物质质量分数的增加,但不同物种对不同CO2浓度升高幅度的响应并不一致(图 3,表 1).对于泥胡菜和网果酸模,叶干物质质量分数对CO2浓度从过去150 μmol/mol升高到当前400 μmol/mol时的响应幅度显著小于从当前400 μmol/mol升高到未来的700 μmol/mol,而野豌豆和藜出现了相反的趋势,但野豌豆的叶干物质质量分数随CO2浓度升高的差异不具有统计学意义;在玉米石中,叶干物质质量分数对将来CO2浓度升高的响应出现负效应(图 3,表 1).
CO2浓度升高促进了网果酸模、野豌豆和藜总干物质质量分数增加,但在网果酸模和藜这2个物种中,总干物质质量分数对过去CO2浓度升高的响应幅度显著小于对将来CO2浓度升高的响应,而野豌豆对不同CO2浓度升高阶段的响应并没有显著差异;泥湖菜的总干物质质量分数随着CO2浓度升高出现了降低的趋势,玉米石总干物质质量分数在过去CO2浓度升高阶段出现了增加趋势,而在将来CO2浓度升高阶段出现了降低趋势(图 3,表 1).方差分析表明,叶干物质质量分数和总干物质质量分数在不同物种间存在差异具有统计学意义,且在不同物种和CO2浓度处理间存在着显著的交互作用(表 2).
2.1. 生物量
2.2. 相对生长速率
2.3. 根质量分数
2.4. 叶干物质质量分数和总干物质质量分数
-
植被生物量是植物碳蓄积的重要组成部分,反映植被生长状况及当地自然环境的变化情况.CO2浓度升高会增加植物的生物量,具有重要的“施肥效应”[36-37].如CO2浓度升高使山羊草和大麦的生物量平均提高40%[2];使大豆的生物量增加40%,种子产量提高30%[38].植物在低CO2浓度下具有较低的生物量积累.在低CO2浓度下,有限的C资源必然会限制光合作用的羧化反应、增加光呼吸、限制植物的生长,从而降低生物量生产[18, 38].已有研究表明,当CO2浓度从目前的350~380μmol/mol降低到180~220μmol/mol时(其他条件均适宜),现代C3植物的生物量降低幅度为40%~70%,平均生物量降低50%.如果CO2浓度继续下降至150μmol/mol,一年生C3植物茼麻Abutilon theophrasti的生物量甚至降低92%[15].在本研究中,CO2浓度升高增加了6种草本植物的生物量,说明CO2浓度升高确实具有显著的肥效作用.然而,当CO2浓度从过去150μmol/mol升高到当前400μmol/mol时,其生物量的增加远远超过从当前400μmol/mol升高到将来的700μmol/mol,且这2个阶段的升高幅度呈相反趋势,即在过去低CO2浓度下受抑制越严重的物种在将来CO2浓度升高时受到的刺激作用越大.Ward等[39]的研究也发现拟南芥Arabidopsis ttiaiiana对低CO2浓度的响应大于对高CO2浓度的响应,可能是植物对光合作用的负反馈限制了碳的获取.Sage等[2]也指出,植物对低CO2浓度响应时碳受到的限制可能会改变植物对外界环境的忍受限度,从而影响植物对未来CO2浓度的响应.这个结果一定程度上证实了我们的假设,即植物对过去低CO2浓度的极度适应将限制其对将来高CO2浓度的响应.
相对生长速率(RGR)是指单位时间内植物干质量的增加量占原有数量的比值,可作为植株生长能力的指标.高CO2浓度通常会促进植物生长,植物具有较高的相对生长速率;在低CO2浓度下植物的净同化速率(NAR:即每单位时间每单位叶面积的生物量变化)降低,叶面积比(LAR:总叶面积与总生物量的比)增加,因此具有较低的相对生长速率.在本研究中,网果酸模和野豌豆的相对生长速率均随CO2浓度升高一直呈增加的趋势,这与Dippery等[15]对茼麻短时间的研究结果相似,主要由NAR的变化所致[15].泥湖菜和藜的相对生长速率在CO2浓度从过去150μmol/mol升高到当前400μmol/mol时显著增加,但当CO2浓度从现在400μmol/mol升高到将来700μmol/mol时,出现了下降趋势,玉米石也同样出现了下降趋势.Celia等[40]在不同CO2浓度下对野胡萝卜相对生长速率进行研究时也得出了相似的结果,当CO2浓度升高到600μmol/mol时,野胡萝卜相对生长速率比CO2浓度为200μmol/mol和330μmol/mol时更低.这一结果也出现在其他物种中[15, 40].这可能是这几种植物对碳、氮的摄取比例不一致,植物体内资源分布不平衡所致[40].但是这3种植物的生物量并没有明显地降低,这与其初始取样时植株较大有关.
根质量分数(RMF)反映的是植物对根系资源分配的比例,是植物适应不同环境条件的重要途径.在CO2浓度升高的条件下,根质量分数通常增加.根质量分数的增加可通过两方面实现:一方面通过减少地上部生长以减少对所需养分和水分的需求;另一方面通过增加根系生长以便吸收更多的养分和水分满足地上部适应不同环境的需求.在本实验中,当CO2浓度从150μmol/mol升高到400 μmol/mol时,所有物种的根质量分数总体上均随CO2浓度升高而增加,说明随着CO2浓度升高,植物光合底物C源变得充足,因此植物将更多的营养物质分配给地下部分,从而有利于土壤中水分和养分的吸收,以满足植物对水分和养分的需求[15].然而,当CO2浓度从400 μmol/mol升高到700 μmol/mol时,除了泥胡菜,其他植物的根质量分数却并没有显著增加.可能原因是这些植物在水分较好和CO2浓度升高条件下,地上部分由于C充足生长速度过快,超过了地下根系的生长,尽管水肥的需求增加,但仍然导致根质量分数的降低.
干物质质量分数(DMC)是反映植物获取资源能力的指标,表示为植物干质量和植物鲜质量的比值,可反映植物生态行为的差异[41].高浓度CO2会影响植物体内碳水化合物的平衡,当碳水化合物的“汇”规模较小或碳水化合物的传输受到限制,则可能会因为过多碳水化合物在叶片中积累,可溶性糖质量分数增多,使叶片干物质质量分数增加;而在低CO2浓度下,植物受C源限制,光合作用减弱,干物质积累减小.在本研究结果中,除泥胡菜以外,其他植物的叶片和总干物质质量分数总体上均随着CO2浓度的升高而增加,说明CO2浓度升高刺激了植物光合物质的积累,也有利于叶片中淀粉的积累[42].但就泥胡菜而言,其叶干物质质量分数随CO2浓度升高逐渐增加,但总干物质质量分数与此相反,随CO2浓度升高反而出现了下降的趋势,说明植物的根系干物质随CO2浓度升高有很大程度的降低.
CO2浓度升高刺激了6种草本植物的生物量积累,即CO2浓度升高具有一般的“肥效”作用.但是这6种草本植物对代表不同地质历史时期内的CO2浓度升高具有不同的响应幅度:生物量和相对生长速率在过去时间内随着CO2浓度升高而升高的幅度远远大于将来时间内的升高幅度,根系生物量的分配在过去时期内随着CO2浓度升高会显著升高,但在将来时间内并没有随着CO2浓度的升高而显著升高.叶干物质质量分数和总干物质质量分数也出现了不一致的响应.研究结果一定程度证实植物对过去低CO2浓度的极度适应将限制其对将来高CO2浓度的响应.






 DownLoad:
DownLoad: