-
过渡金属配合物具有优秀的催化活性和良好的反应选择性,广泛应用于天然产物、药物以及功能材料合成等诸多领域[1-3]. 镍配合物相对于同族钯配合物具有低毒性和廉价的特点,且具有氧化态多变、强配位、易与亲电试剂反应等独特的化学活性,在过去几十年中引起了科研工作者的广泛关注. 功能性官能团取代的有机羧酸配体和金属配位组装合成的金属羧酸配位化合物,作为一类新兴多孔材料,因其在能源气体吸附分离、不对称催化、磁学、荧光和化学传感等众多领域有潜在应用,其设计合成、晶体结构和性质研究成为国际国内研究热点[4]. 磺酸与羧酸较为相似,且磺酸基相比于其他基团最突出的优点在于:磺酸基的氧原子与金属离子的配位方式灵活多样,配位结构丰富多变,容易形成高维结构,而且磺酸基的氧原子也是较好的氢键的受体,可以通过氢键形成一维链状、二维层状和三维网状结构. 近年来的研究发现,含磺酸基配合物具有卓越的催化性能[5-9].
金属磺酸盐作为一种新型的耐水性催化剂,具有较高的Lewis酸性,在Biginelli反应中,酸催化剂能够激活芳醛中亲电性不强的羰基,使其亲电性增强,从而与脲中亲和性较弱的氨基反应. 由此,金属磺酸盐配合物具有良好的催化活性,例如王敏等[10]采用对甲基苯磺酸铝为催化剂,高效合成了一系列4,6-二芳基-3,4-二氢嘧啶-2(1H)-酮,反应时间短,产率高,催化剂廉价易得且可重复使用. 此外,金属磺酸盐配合物在电镀[11-12]和电化学[13-14]等方面也有着广泛的应用. 因此,对金属磺酸盐配合物的结构及催化性能的研究是很有必要的.
本研究合成了对甲基苯磺酸镍,采用溶剂挥发法得到配合物晶体后对其结构进行了表征,并以Biginelli反应为探针研究了其催化性能.
HTML
-
熔点用RD-Ⅱ型熔点仪(天津市天光仪器公司)测定,温度计未校正;红外光谱用Scimitar 2000 Near FTIR Spectrometer型傅里叶变换红外光谱仪(美国Agilent公司)测定,KBr压片,测试范围为400~4 000 cm-1,光谱分辨率为4 cm-1;美国Perkin-Elmer公司PYRIS DIAMOND TG/DTA热分析仪,温度范围为30~880 ℃,程序升温速率为10 ℃/min,流速为20 mL/min;德国Bruker公司Bruker SMART APEX Ⅱ型单晶X-射线衍射仪,以ω-2θ扫描方式收集数据,Mo-Ka射线(λ=0.071 073 nm)为辐射源,所有数据经Lp因子校正.
所有试剂均为市售分析纯或化学纯.
-
称取对甲基苯磺酸120 mmol,用50 mL水溶解后加入到反应瓶中,缓慢加入65 mmol的碱式碳酸镍,在搅拌条件下加热回流1 h. 趁热过滤,然后将滤液蒸干,干燥得到绿色的对甲基苯磺酸镍,分离产率为91.8%. 将对甲基苯磺酸镍在室温下溶解在水中,采用溶剂挥发法,静置数天后得到绿色块状的对甲基苯磺酸镍晶体.
-
选取尺寸大小合适的晶体,置于单晶衍射仪上,采用Mo-Kα射线(λ=0.071 073 nm)作为辐射源,在296(2)K以ω-2θ扫描方式收集衍射数据. 所有数据经Lp因子校正,未作吸收矫正. 数据还原和结构分析采用SHELXS-97程序完成,由直接法进行结构解析,非氢原子由差值Fourier法指认,氢原子由差值Fourier法和理论加氢法联用确定,氢原子参与结构因子的计算,但不参与修正. 经解析后的配合物的晶体学数据、部分键长键角和氢键数据展示在表 1和表 2中. 详细数据已存储于英国剑桥晶体学数据库(CCDC编号为1986376).
1.1. 仪器与试剂
1.2. 对甲基苯磺酸镍配合物的合成
1.3. 配合物的单晶结构检测
-
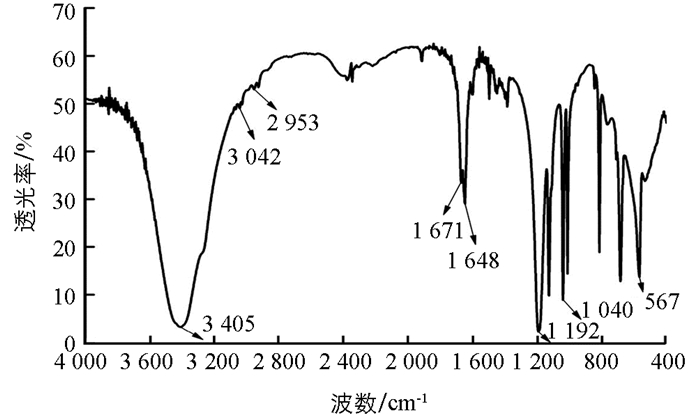
对甲基苯磺酸镍的红外光谱见图 1. 苯环的骨架振动特征峰在1 671 cm-1处;3 042 cm-1处的峰为苯环上的C—H伸缩振动的特征峰;1 192 cm-1和1 040 cm-1的峰为磺酸基的硫氧双键的特征峰;1 192 cm-1处的特征峰是磺酸基的硫氧双键的不对称伸缩振动;甲基中C—H的伸缩振动特征峰在2 953 cm-1处;Ni—O的特征峰在567 cm-1处;在3 405 cm-1和1 648 cm-1处的峰为O—H键的伸缩振动和弯曲振动的特征峰,说明对甲基苯磺酸镍中存在水分子.
-
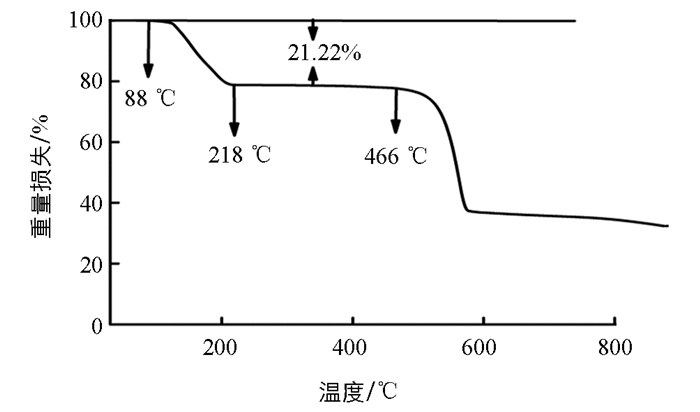
对甲基苯磺酸镍的热重谱图见图 2,对其进行热稳定性检测. 测试结果表明,对甲基苯磺酸镍在30~880 ℃内的失重分为两个阶段,第一阶段的失重是在88~218 ℃范围内完成的,该段是由失去配位水引起的失重. 对甲基苯磺酸镍的失水率为21.22%,理论值为21.21%,理论值与实验值基本一致. 推断对甲基苯磺酸镍含有6个结晶水. 第二阶段的失重发生在466 ℃后,这一阶段失重较大,可能是因为对甲基苯磺酸镍中的苯磺酸分子骨架坍塌引起的失重.
-
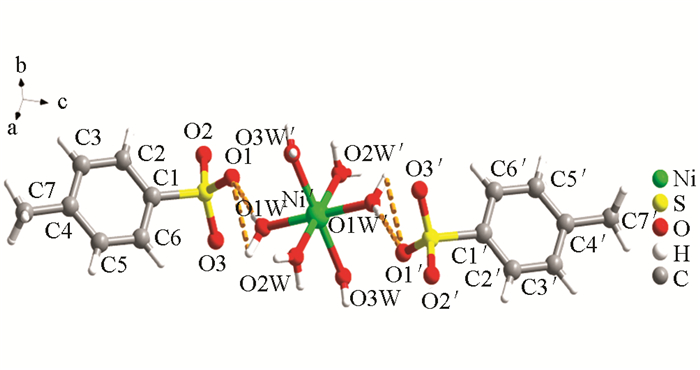
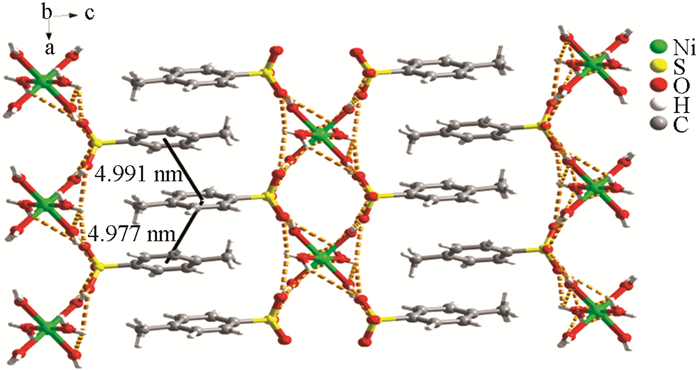
采用X-射线单晶衍射法对其配位方式和空间结构进行测试,测试结果表明对甲基苯磺酸镍属于单斜晶系P21/n空间群. 如图 3所示,在配合物[Ni(H2O)6](p—CH3C6H4SO3)2的分子结构中,Ni2+离子分别与6个配位H2O分子中的O原子配位,其中NiO的键长(表 2),Ni(1)—O(1W)与Ni(1)—O(1W′)键长相等为0.204 3 (5) nm,Ni(1)—O(2W)与Ni(1)—O(2W′)键长相等为0.202 4 (4) nm,Ni(1)—O(3W)与Ni(1)—O(3W′)键长相等为0.204 9 (5) nm. 轴向的键角O(1W)′—Ni(1)—O(1W)、O(2W)—Ni(1)—O(2W)′、O(3W)′—Ni(1)—O(3W)(#1:-x-1,-y+1,-z+1)均为180°. 但是这3个轴向之间的夹角均不为90°,这些数据表明Ni2+与6个配位水所形成的阳离子基团为六配位的畸形八面体结构. 对甲基苯磺酸基团仅以抗衡阴离子的形式存在,并未与中心金属离子发生配位,由分子内氢键进行连接(O1W—H1WA···O1,0.205 00 (2) nm;O1W—H1WB···O1,0.317 94 (4) nm;O1W—H1WA···O1,0.205 00 (2) nm;O1W—H1WB···O1,0.317 94 (4) nm). 在对甲基苯磺酸镍分子层与层之间以水为氢键供体,磺酸基作为氢键受体,在氢键和π-π共同作用下堆砌成的三维层状结构(图 4).
-
以Biginelli反应为探针,考察对甲基苯磺酸镍的催化性能,在80 ℃、无溶剂条件下,以对甲基苯磺酸镍(0.5 mmol)为催化剂,苯甲醛(10 mmol)、1,3-二羰基化合物(乙酰乙酸甲酯、乙酰乙酸乙酯)(10 mmol)和脲(甲基脲、尿素)(13 mmol)通过三组分“一锅法”合成了3,4-二氢嘧啶-2-(1H)-酮衍生物,反应方程式为
由表 3可知,对甲基苯磺酸镍对Biginelli反应具有较好的催化性能(表 3,序号1-3),推测催化剂结构对催化活性有一定影响. 从图 3可以看出,对甲基苯磺酸镍的金属离子与水配位后以水合阳离子形式存在,相比于其他金属配合物中金属离子与磺酸配体直接配位空间位阻小,所以更易参与到反应中;同时,磺酸基团有较强的氢键形成能力,与配位水形成氢键,从而削弱中心离子与水的配位能力,增强Lewis酸性,提高催化能力. 实验还考察了苯甲醛、乙酰乙酸乙酯和甲基脲在无催化剂条件下的反应,5 h后才得到较少的产品(表 3,序号4).
关于催化机理,笔者认为首先醛和脲在催化剂的作用下进行缩合反应生成N-酰基亚胺中间体1,该中间体进一步与酯2进行加成反应得到开链的酰脲3,3再环化、脱水得到相应的目标产物4[16-17],可能的催促作用机理为
2.1. 对甲基苯磺酸镍的红外光谱表征
2.2. 对甲基苯磺酸镍的热重表征
2.3. 对甲基苯磺酸镍的晶体结构
2.4. 对甲基苯磺酸镍的催化性能研究
-
合成了对甲基苯磺酸镍配合物,通过红外光谱、热重和X-射线单晶衍射明确了其结构和热稳定性. 该配合物属于单斜晶系,P2(1)/n空间群,中心镍离子和6个配位水所形成的阳离子基团,是畸形的八面体空间结构,对甲基苯磺酸基团仅以抗衡阴离子的形式存在,并未与中心金属离子发生配位,由分子内氢键进行连接. 以Biginelli反应为探针,证明对甲基苯磺酸镍的催化性能较好,并对催化剂结构与催化活性间的关系进行了推测.






 DownLoad:
DownLoad: