-
黄连为毛茛科黄连属植物黄连(Coptis chinensis Franch.)、三角叶黄连(Coptis deltoidea C.Y.Cheng et Hsiao)或云连(Coptis teeta Wall.)的干燥根茎. 史载于《神农本草经》,列为上品. 性寒,味苦,归心、脾、胃、肝、胆、大肠经. 具有泻火解毒,清热燥湿,用于湿热痞满、呕吐吞酸、高热神昏、心火亢盛、心烦不寐、心悸不宁等[1]. 是我国内销和出口的大宗药用植物,也是全国100多种中成药的原料,主产于四川、湖北、重庆等地. 近10年来,根腐病在各大产区爆发,使连农遭受重大损失,种连积极性大大降低,严重制约着黄连产业的规模化发展. 此外,黄连连作会加剧根腐病的发生[2-3]. 该病目前还没有有效的防治方法.
项目组和其他研究人员前期研究的结果表明,真菌是黄连根腐病的致病病原之一,其中镰刀菌是强致病菌[4-5]. 同时,病根中腐霉属真菌分离频率也很高,高于镰刀菌[6],推测其中可能也包含致病菌,因此本研究对其中的腐霉属真菌进行了致病性研究.
HTML
-
材料和取材方法见已发表文献[6].
-
B518259Ezup柱式真菌基因组DNA抽提试剂盒,生工SangonBiontech;RR178 Fungi Identification PCR Kit,Takara;马铃薯葡萄糖琼脂培养基,北京陆桥;脱皮麦粒-木屑培养基:木屑和脱皮麦粒清水浸泡24 h,沥干水分,按照木屑与脱皮麦粒比例为3∶1混合均匀,装平皿,121 ℃灭菌40 min.
超微量分光光度计(NanoDrop 2000),Thermo Scientific公司;水平电泳仪(DYY-6C),北京六一生物科技有限公司;PCR仪(PTC-100),Bio-Rad公司;凝胶成像仪(Gel Doc XR),Bio-Rad公司;电子显微镜(OlympusCX31-32C02),奥林巴斯株式会社.
-
组织分离的方法见已发表的文献[6],25 ℃培养5~7 d后,挑取组织周围长出的似腐霉属的菌丝(菌丝白色,短,稀疏,贴培养基表面生长,菌落呈菊花瓣型、月季花瓣型、放射型或平滑型[7-8]),转接入PDA中进行纯化,直到获得纯菌落.
-
分子鉴定的方法见已发表的文献[6].
-
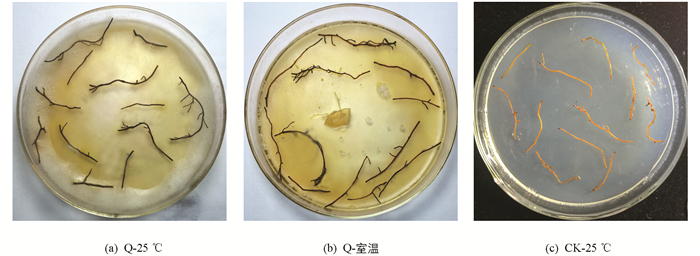
选取腐霉菌株,在PDA培养基上,黑暗条件下,25 ℃培养5~7 d,至菌丝长满平皿. 剪取健康黄连根条(约3~4 cm),无菌水清洗5次,无菌滤纸吸干多余水分,置于平皿中菌丝上,12条根/平皿,分别置于25 ℃和室温(4.3~14.2 ℃),在黑暗条件下共培养7~10 d后统计发病情况. 以25 ℃下水琼脂培养基上培养的健康黄连根条为对照.
-
取离体回接筛选出的发病严重的菌株,接种于脱皮麦粒-木屑培养基上,25 ℃黑暗条件下培养10~15 d,至菌丝长满培养基,备用;健康2年生黄连苗,将根部泥土杂物洗净,无菌水清洗5次,剪断部分须根制造伤口,备用;河沙混合腐殖土(2∶1)作为基质,120 ℃高温烘烤灭菌3 h,冷却,加无菌水润湿,备用;小花钵用5 ‰高锰酸钾溶液浸泡24 h消毒后用无菌水洗净,先加入厚约2 cm的灭菌基质垫底,再填入2 g长满腐霉菌丝的脱皮麦粒-木屑培养基,栽入黄连苗,把基质和脱皮麦粒-木屑菌种混合均匀后填入花钵盖住根,浇透无菌水,置于25 ℃培养箱,光照8 h /d. 部分苗置于室温生长(日均温4.7~11.2 ℃). 12株苗/处理. 对照苗不接种菌,其余培养方法同上. 7 d后开始观察,15 d后统计发病情况,计算病情指数(DI). 并取发病根条,按照步骤1.3.1的操作进行病原菌再分离.
式中,PA为各级病株数,LE为级数,TO为调查总数,HI为最高级数.
-
以致病菌株ITS序列为基础,用BioEdit和MEGA6.0软件按照邻接法,自展数为1 000,构建系统发育树.
-
取致病菌株,在PDA培养基上采用“插片法”,25 ℃黑暗培养10~15 d后,进行显微形态观察.
-
由于腐霉在PDA上长期保存会很快枯萎死亡,不容易继代. 因此对其保存培养基进行了筛选. 基质原料:带皮小麦(浸泡48 h过心,沥干水分)、脱皮小麦(浸泡48 h过心,沥干水分)、木屑(浸泡24 h,挤干水分)、玉米油,以上原料按照不同比例进行混合后,装平皿,121 ℃灭菌40 min,接种腐霉菌种,25 ℃培养15~20 d后,观察菌丝生长状况,筛选最优良的培养基.
1.1. 材料
1.2. 试剂与仪器
1.3. 方法
1.3.1. 腐霉属菌株分离与纯化
1.3.2. 可培养真菌分子鉴定
1.3.3. 离体回接
1.3.4. 活体回接
1.3.5. 致病菌系统发育分析
1.3.6. 病原菌形态观察
1.3.7. 腐霉培养基筛选
-
共计分离到8个不同的腐霉属菌株. 由于其他菌株污染,取未污染的菌株Q进行离体回接. 结果表明,无论是高温还是低温下,它的致病性都比较强,大约5 d后开始发病,10 d时根条全部腐烂变黑. 因此挑取Q进行进一步的活体回接试验(表 1,图 1).
-
Q菌株在室温和25 ℃下均会发病,发病程度高温比低温略高,差别不明显. 病株全部根或大量根变黑,叶片枯萎,新叶较对照数量少,长势弱. 活体回接发病的根条进行再分离,仍然能分离到形态相同的菌株(表 2,图 2).
-
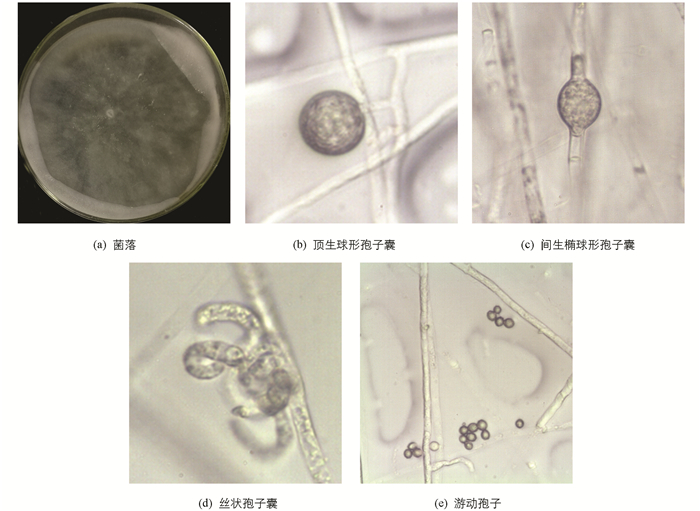
Q菌株的菌落在PDA上呈玫瑰花瓣形,菌丝短,米白色,贴培养基表面生长,较稀疏. 菌丝直径3.1~6.7 μm左右. 孢子囊有丝状、球形或椭球形两种,后者直径17.7~20.4 μm. 游动孢子直径3.8~6.6 μm,部分发现芽管萌发[7](图 3).
-
培养基筛选接种的菌种是Q,它在纯带皮麦粒和纯木屑上几乎无菌丝生长,而纯脱皮麦粒上生长最旺盛,可作为腐霉生长和保存的培养基. 添加玉米油对菌丝生长有一定的促进作用(表 3).
-
8个腐霉属菌株的分子鉴定结果见表 4.
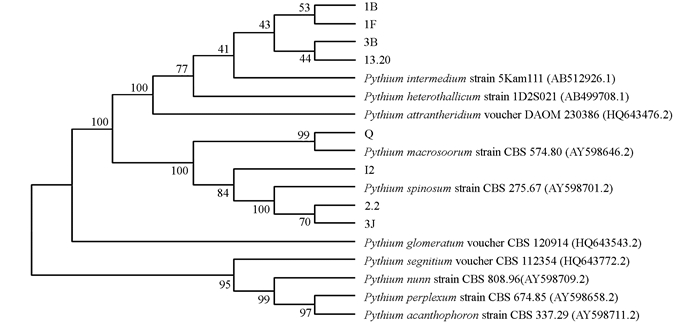
系统发育分析结果表明,1B,1F,3B,13.20和P. intermedium聚为一枝,I2,2.2,3J和P. spinosum聚为一枝. Q和P. macrosporum strain CBS 574.80聚为一枝(图 4).
2.1. 离体回接发病情况
2.2. 活体回接发病情况
2.3. 致病菌株显微形态
2.4. 最适合培养基筛选
2.5. 腐霉属菌株分子鉴定结果及致病菌系统发育分析
-
腐霉(Pythium)是藻物界,卵菌门,卵菌纲,霜霉目,腐霉科的一个属[8]. 该属真菌分布十分广泛,且可腐生、寄生或兼性寄生,又可水生、陆生或水陆两栖生. 许多种类是重要的土传植物病原菌,常造成果实腐烂、根腐、茎基腐和幼苗猝倒病等. 迄今为止,重要的植物病原腐霉已经超过80种,对农作物危害很大[9].
腐霉属菌在黄连根腐病根条中分离频率很高,且包含多个种[6]. 这些种很多都是其他植物的根腐病菌. P. spinosum可导致小麦根腐病[10],P. sylvaticum可导致大豆、大蒜根腐病[11-12]. P. intermedium可导致欧防风(Pastinaca sativa L.)[13]、狗蔷薇(Rosa canina L.)[14]幼苗根腐病. P. macrosporum可导致花卉鳞茎腐烂和草类[15]、胡萝卜[16]的根腐病. Q和P. macrosporum strain CBS 574.80聚类可信度是100,P. macrosporum strain CBS 574.80是模式菌株,A.J.van der用这株菌对P. macrosporum这个种进行描述和定义[7],结合显微特征,说明Q就是P. macrosporum. 因此P. macrosporum是黄连根腐病致病菌之一. 本研究还发现该菌在低温下也对黄连根条具有致病性,且致病能力和25 ℃时没有明显差别. 其他腐霉也有类似报道,如Pythium sulcatum在10 ℃,18 ℃和24 ℃均能导致欧芹幼苗[13]和狗蔷薇幼苗根腐病[14]. 国内外有关P. macrosporum的报道很少,该菌在荷兰[7, 15]、德国[7]、加拿大[7, 16]和美国[17]均有分离到,在国内是首次分离报道. 根据以上分析推测,其他7株未作回接的腐霉菌株中可能也包含在不同温度侵染黄连根部的致病菌,有待进一步研究.
腐霉离体回接发病表现和镰刀菌不同,前者只是腐烂变黑,而后者会产生大量黄色水珠,且腐烂程度比腐霉高[5]. 说明腐霉致病能力不如镰刀菌强. 而即便如此,腐霉具有镰刀菌不具备的低温致病能力[5],与高温致病的镰刀菌配合,延长了侵染时期,大大增加了黄连根腐病的防治难度.
腐霉菌丝在纯带皮小麦上几乎不生长,加了湿润的木屑才生长,在脱皮小麦上生长最好,推测原因是纯带皮小麦表面水分容易干,木屑和脱皮小麦能保持湿润. 但腐霉在纯木屑上不生长,说明它对木质纤维分解能力弱,需要淀粉作为外源养分. 很多腐霉的培养基都含有油脂类成分,常用的KPYG2培养基就含有玉米油[18-19]. 因此,本研究在基质中添加玉米油,结果对菌丝生长有一定促进作用. 推测玉米油和纯脱皮小麦的组合培养效果更佳,拟进行进一步的比较研究.







 DownLoad:
DownLoad: