-
开放科学(资源服务)标志码(OSID):

-
果胶酶广泛存在动植物和微生物中,其中聚半乳糖醛酸酶(Polygalacturonase,PG)是最早发现且应用广泛的一种果胶水解酶,可通过水解作用切断果胶酸分子的α-1,4糖苷键,将果胶降解为小分子[1]. 微生物由于生长速度快,常用于酶的生产. 已报道的产聚半乳糖醛酸酶的菌种主要为曲霉[1-2]、木霉[3]和青霉[4-5],也有细菌[6]和酵母菌[7]等. PG可用于果汁和果酒生产,Patil等[8]用分离的拟青霉发酵生产PG,显著降低了果汁样品的黏度,在果汁澄清中有重要作用. 柑桔加工会产生大量的皮渣,产果胶酶和纤维素酶的菌株可以用于降解柑桔皮渣[9]以及发酵生产柠檬酸[10]. 柑桔果皮因富含果胶被较多用于提取柑桔果胶[11]. 柑桔果胶可以通过果胶酶酶解生产果胶低聚糖[12],也可利用PG通过水解作用切断果胶酸分子的α-1,4糖苷键,将果胶降解为分子量较小的低聚糖类,得到果胶低聚糖. 果胶低聚糖具有低热量、抗肿瘤、抗氧化、抑菌等作用[13],具有较好的应用价值. Olano-martin等[14]、丁长河等[15]用PG分别水解柑桔果胶、苹果果胶等可得到果胶低聚糖. 虽然PG产酶菌种和酶学性质研究较多,但聚焦于挖掘适用于利用柑桔皮渣果胶制备果胶低聚糖的新型耐酸性PG及其产酶菌株的研究较少.
根际土壤中富含微生物[16],本研究从柑桔果园土壤中筛选到能产聚半乳糖醛酸酶的一株真菌GYS-6,经分子生物学和形态学鉴定为皮落青霉,对发酵条件进行优化以充分发挥菌株的产酶性能,对该酶的酶学性质及酶谱特性进行研究,并将该酶用于酶解柑桔果胶,经薄层层析和高效液相色谱分析确定可得到果胶低聚糖,以期为聚半乳糖醛酸酶和果胶低聚糖的生产提供研究基础.
HTML
-
菌株GYS-6,从柑桔的土壤中分离筛选获得,中国微生物菌种保藏中心登记号为CGMCC 3.15886.
-
试验用到的试剂与培养基主要有:D-半乳糖醛酸、聚半乳糖醛酸,美国sigma; 柑桔果胶,上海阿拉丁; PDA培养基、酵母膏、NaCl、KH2PO4、K2HPO4、MgSO4、蔗糖、葡萄糖、硫酸铵、氯化铵、硝酸铵、尿素、Tris、HCl、SDS、考马斯亮蓝R-250、甲醇、冰乙酸,均为国产分析纯; 乙腈,Sigma-Aldrich.
菌种的鉴定采用麦芽汁培养基,菌种的活化采用PDA培养基,菌种的发酵采用发酵培养基:桔皮粉10 g/L、酵母膏10 g/L、NaCl 2.0 g/L、KH2PO4 0.3 g/L、K2HPO4 1.0 g/L、MgSO4 0.3 g/L,自然pH值.
-
光学显微镜,日本Olympus公司生产; PCR仪easycycler 96,德国耶拿分析仪器股份公司生产; 紫外分光光度计TU-1901,北京普析通用仪器有限责任公司生产; 高速冷冻离心机TGL-20M,湖南湘仪实验室仪器开发有限公司生产; 电泳仪Bio-Rad,美国伯乐公司生产; 透析袋:MD1425-5m 8000-14000,上海源叶生物科技有限公司生产; 高效液相色谱仪,美国DIONEX公司生产.
-
以D-半乳糖醛酸含量(μmol)为横坐标x,吸光值为纵坐标y,所得标准曲线为y=0.586 3x-0.366,R2=0.996 6.
-
发酵后产物过滤后经冷冻离心机1 000 r/min离心20 min,取上清液测定聚半乳糖醛酸酶活力[17].
酶活力测定[18-19]:采用改良后的DNS法,以D-半乳糖醛酸做标准曲线; 以灭菌后酶液作为对照,540 nm比色,每分钟催化底物产生1 μg半乳糖醛酸所需酶量定义为1个酶活力单位,以U/mL表示.
-
将菌种在麦芽汁培养基中培养10 d,观察菌落形态、菌丝、分生孢子等,根据《真菌鉴定手册》进行形态学的鉴定[20].
β-微管蛋白(TUB)测定基因序列. 上游引物:5'-AATI'GGTGCCGCTTTCTGG-3',下游引物:5'-AGTTGTCGGGACGGAATAG-3'. PCR体系:总体积30 μL,其中2×easyTaqSuperMix 15 μL,上、下游引物各1 μL (10 pmol/μL),模板(提取的真菌DNA)3 μL(100~120 ng/μL),加双蒸水补至30 μL. 反应条件为94 ℃ 5 min预变性,然后94 ℃ 30 s,55 ℃ 45 s,72 ℃ 1 min共35个循环,72 ℃延伸10 min. 扩增产物用1%琼脂糖凝胶电泳检测并切取目标片段,送华大基因测序,将测序所得序列在NCBI上进行BLASTS比对分析[21-22].
-
酶的初步纯化采用硫酸铵分级沉淀及盐析[23]. 将粗酶液缓缓加入硫酸铵粉末至饱和度0%~20%,20%~40%,40%~60%,60~80%. 沉淀过夜后冷冻离心机12 000 r/min离心30 min,收集沉淀,用0.2 mol/L醋酸(pH值5.0)缓冲液复溶,用透析袋(8 000~14 000)透析,缓冲液做透析液,定期更换透析液,直至氯化钡检测透析液中无沉淀为止,透析袋内液体即为初步纯化酶,测定其聚半乳糖醛酸酶活力,计算盐析后各段酶活占总酶活的比例.
采用十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)[24],酶谱样品不加热,并且在分离胶中加入0.1%聚半乳糖醛酸. 电泳完成后,用2.5%的Triton X-100缓冲液浸泡1 h,再37 ℃缓冲液孵化24 h,用蒸馏水漂洗后,用0.05%钌红染色1 h,蒸馏水漂洗.
-
将初步纯化聚半乳糖醛酸酶在不同温度(25~70 ℃)下测定酶活,以酶活最大值时酶活力定义为100%,计算不同温度下的相对酶活力(即不同温度条件下的酶活性占最高酶活性的百分比). 将酶液在不同温度(25~55 ℃)条件下保温30 min后立即冰浴,待冷却后测定酶活,计算剩余酶活性与未处理时酶活性比值作为相对酶活力.
-
在温度45 ℃时,将酶液置于不同pH值缓冲液(pH值3.0~8.0)下测定酶活,以酶活最大值时酶活定义为100%,计算不同pH值条件下相对酶活力(即不同pH值条件下的酶活性占最高酶活性的百分比). 将酶液置于不同pH值缓冲液(pH值2.2~10.86)下保持30 min后,测定酶活,计算剩余酶活性与未处理时酶活性比值作为相对酶活力.
-
在0.1 mmol/L,0.5 mmol/L,1 mmol/L,5 mmol/L和10 mmol/L下,测定金属离子(K+,Na+,Ca2+,Mg2+,Ba2+,Fe2+,Zn2+,Mn2,Cu2+,Co2+,Pb2+,Al3+)对聚半乳糖醛酸酶活性的影响,以不添加金属离子时的酶活计为100%,计算添加金属离子后的相对酶活力.
-
酶解果胶生产果胶低聚糖:用醋酸缓冲液(pH值4.5)配置2.0%的果胶溶液和1 U/mL初步纯化酶液,体积比按2∶3,在40 ℃恒温水浴磁力搅拌下进行酶解,分别酶解0 min,5 min,10 min,30 min,60 min,90 min,270 min后,立即取出沸水浴灭活15 min,流动水冷却.
酶解产物经氮吹仪浓缩后进行薄层色谱分析(TLC),在距离薄层板0.5 cm高度处点样,展开剂为正丁醇-乙酸-水(体积比4∶3∶1)混合液,展至距上沿0.5 cm处停止. 染色剂为乙醇-硫酸(体积比9∶1),薄层板吹干后,放入110 ℃烘箱烘烤5 min,冷却观察.
将果胶酶解270 min后样品,用80%乙醇沉淀多糖,上清液真空浓缩后进行真空冷冻干燥,得到果胶低聚糖样品,并进行高效液相色谱(HPLC)分析,示差检测器,色谱柱为氨基柱,流动相为70∶30(乙腈∶水),流速1.3 mL/min,柱温44 ℃,进样量10 μL.
-
本试验所有样品平行测定3次,结果以平均值±标准差表示. 数据用SPSS 17.0进行统计学分析,多组间采用One way ANOVA进行Duncan法比较,p<0.05时认为差异有统计学意义. 折线图和柱状图用origin 8.5进行绘制.
1.1. 菌株
1.2. 试剂与培养基
1.3. 仪器与设备
1.4. 试验方法
1.4.1. 聚半乳糖醛酸标准曲线
1.4.2. 聚半乳糖醛酸酶活力测定方法
1.4.3. 菌种的鉴定
1.4.4. 酶的初步纯化及酶谱分析
1.4.5. 酶的最适温度及温度稳定性[25]
1.4.6. 酶的最适pH值及pH值稳定性[25]
1.4.7. 金属离子及其浓度对酶的活力影响[26]
1.4.8. 果胶水解制备低聚糖应用
1.5. 数据处理
-
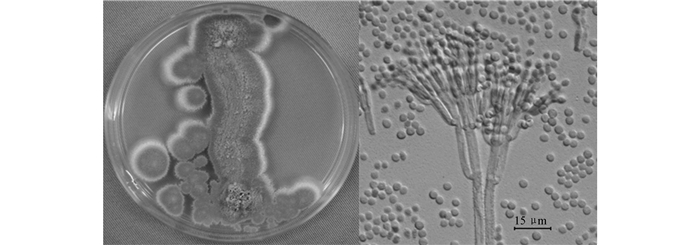
通过单因素优化试验发现最佳碳源为柑桔皮果胶,说明该菌株产酶为诱导型,优化后聚半乳糖醛酸酶活力从0.79 U/mL提高至3.59 U/mL. 菌种在麦芽汁培养基上生长较快,25 ℃黑暗条件下培养10 d,菌落直径35~40 mm,边缘白色,中部灰绿色,平铺,气生菌丝稀少; 分生孢子结构大量产生,质地绒兼粉末状或近颗粒状,后期层状脱落; 背面浅黄褐色,色素不溶于培养基中. 分生孢子结构大量,帚状枝多见3轮生,排列紧密. 分生孢子梗壁粗糙,无色; 瓶梗柱状,颈部明显,(7.5~12.3) μm×(2.0~3.5) μm; 分生孢子球形或近球形,光滑,串生,2.8~4.0 μm(图 1).
β-微管蛋白(TUB)测定基因序列,基因序列测定结果为:5'AAAGCTTGCCGAAGGGACCGGAGCGGACAGCGTCCATGGTACCAGGCTCCAAATCGACGAGAACGGCACGGGGAACGTACTTGTCACCGCTGGCCTAGATTATCAAGGAAAACATCCGATCAGATGATGCACTATTATTCGGTTTCCTGTCGTTGGACTCACATGGTTGAAGTAGACGTTCATACGCTCGAGCTGGAGGTCGGAGGTACCATTGTACCTAGGAAGATATCAGATGTGTAATCCACCGGAAACCCCTATCACTGTTAAAACTTACTGTCCATCGCCATCGAGACCGTGCTCGCCAGAGATGGTTTGCCTGTAATCCAGTTAGGAACCTGTCAATTGATACCCAACGCGAAAAAAAAAAGCTCGGCACTTACCAGAAAGCAGCACC-3'. 将序列在NCBI上进行BLASTS比对分析[21-22],结合形态结构[20],该产聚半乳糖醛酸酶的菌种鉴定为皮落青霉(Penicillium crustosum).
-
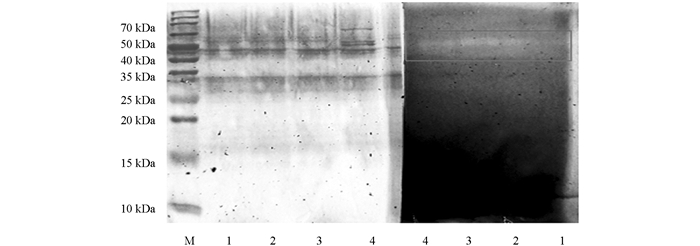
硫酸铵百分比在0%~20%,20%~40%,40%~60%,60%~80%时,酶活比例分别为(2.58±0.02)%,(2.89±0.01)%,(30.46±1.04)%,(64.06±1.01)%; 可见硫酸铵比例低于40%时,聚半乳糖醛酸酶酶活所占比例很低,仅占总酶活的5.47%,聚半乳糖醛酸酶活力以硫酸铵沉淀40%~80%较高,尤其是比例为60%~80%时表现为更高的活力. 皮落青霉产聚半乳糖醛酸酶与商业果胶酶在48 kDa处有明显聚半乳糖醛酸酶活性(图 2). Kester等[27]从黑曲霉中发酵分离得到的2种多聚半乳糖醛酸酶分子量分别为55 kDa和38 kDa,倪鸿静[28]从皮落青霉发酵液中分离得到的聚半乳糖醛酸酶分子量为38 kDa.
-
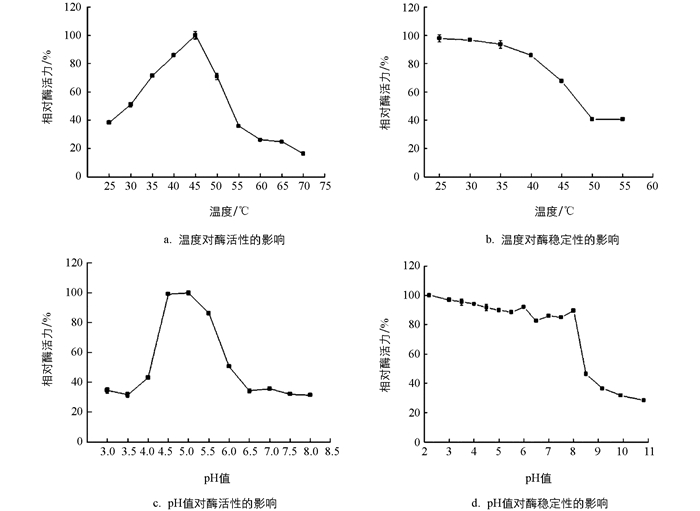
从图 3a可知,皮落青霉产聚半乳糖醛酸酶的最适反应温度为45 ℃. 从图 3b可知,在25~40 ℃下,稳定性较好,40 ℃保温30 min仍能保持85.79%的酶活,45 ℃下保温30 min能保持67.53%的酶活. 从图 3c可知聚半乳糖醛酸酶最适pH值为5.0,其次为4.5. 张名爱等[29]从草酸青霉中分离的PG酶最适温度40 ℃,最适pH值5.0. Kester等[27]研究发现黑曲霉产生的5种聚半乳糖醛酸酶的最适pH值在4.3~4.9的范围内. 从图 3d可知在pH值2.2~8.0保持30 min,聚半乳糖醛酸酶酶活保留率都在80%以上,表明此酶耐酸性好,可适用于苹果、柑桔等酸性果汁加工生产.
-
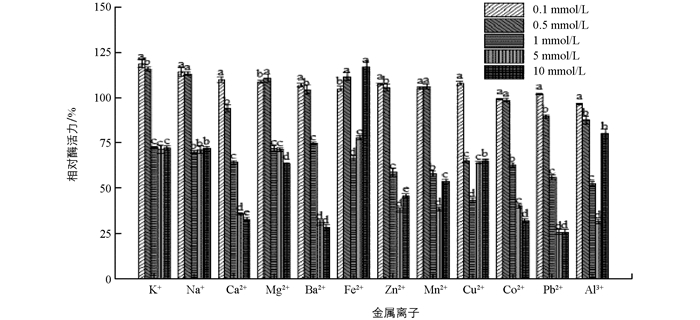
在0.1 mmol/L,0.5 mmol/L下,K+,Na+,Mg2+,Ba2+,Fe2+,Zn2+,Mn2+对聚半乳糖醛酸酶有激活效果,K+激活作用最强,0.1 mmol/L,0.5 mmol/L相对酶活力为118.70%,115.72%. 在1 mmol/L,5 mmol/L,10 mmol/L下,各金属离子均呈抑制作用,其中Fe2+在10 mmol/L呈现激活表现,分析可能跟Fe2+在高浓度有颜色有关(图 4). 唐湘华等[30]对米曲霉产果胶酶添加金属离子浓度5 μg/g,50 μg/g,500 μg/g,1 000 μg/g,发现Pb2+和Ag+严重抑制该酶活力,Cu2+抑制此酶,Mg2+、K+、Na+对酶无明显激活或抑制作用,Ca2+有较弱的激活作用. Kanga等[31]从蜗牛消化液中提取纯化出的2种多聚半乳糖醛酸酶(46 kDa和86 kDa)发现Ba2+是PG2的激活剂,Mn2+,Ca2+,Zn2+是抑制剂.
-
分子量越小,在薄层板上移动越快,试验结果看出,A和B的分子量高于半乳糖和蔗糖,判断其为低聚糖,表明皮落青霉所产聚半乳糖醛酸酶可用于生产果胶低聚糖. 1号为单糖半乳糖,2号为双糖蔗糖,3~8号样品为低聚糖,酶解时间分别为5 min,10 min,30 min,60 min,90 min,270 min. 随着酶解时间延长,A和B颜色也逐渐加深,表明随着酶解时间的延长,低聚糖的产量逐渐增加(图 5).
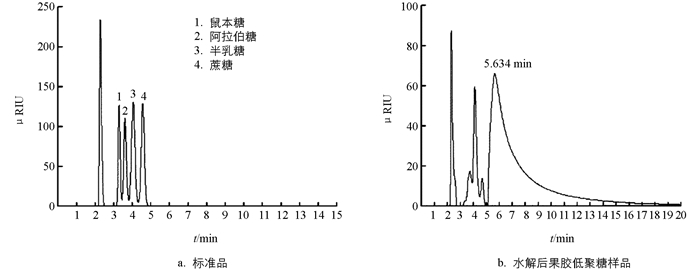
鼠李糖、阿拉伯糖、半乳糖为单糖,蔗糖为双糖,在氨基柱上,随着分子量的增大,流出时间延长. 4种标样的流出时间分别为:1-鼠李糖4.252 min,2-阿拉伯糖4.664 min,3-半乳糖5.241 min,4-蔗糖5.901 min,见图 6a. 柑桔果胶经270 min PG酶酶解,除去多糖后液相色谱图见图 6b,有3个单糖小峰,其余峰主要集中在5.634 min后,双糖蔗糖的峰在5.901 min,表明所得糖主要为二糖以上的低聚糖.
2.1. 产酶条件及菌种鉴定结果
2.2. 硫酸铵分级沉淀及酶谱分析
2.3. 酶的最适温度和热稳定性、最适pH值和稳定性
2.4. 金属离子对酶活的影响
2.5. 聚半乳糖醛酸酶酶解果胶
-
本研究通过菌种的产酶筛选、菌种鉴定、发酵条件优化、酶学特性、酶谱分析及酶的应用等分析,获得一株新型产聚半乳糖醛酸酶菌株皮落青霉GYS-6,经过柑桔果胶诱导可以生产耐酸性较强的聚半乳糖醛酸酶. 单因素试验优化发酵条件将聚半乳糖醛酸酶活力从0.79 U/mL提高至3.59 U/mL. 后期若要重复使用提高工业化应用,可采用海藻酸钙包覆的聚吡咯/银纳米复合材料固定PG的方法[32]. 将粗酶用硫酸铵沉淀并用透析袋进行透析后得到初步纯化酶,对其酶学特性进行研究发现,该酶最适温度45 ℃,最适pH值为5.0,低浓度的K+等离子对酶活有一定激活作用,浓度高于1 mmol/L对酶活有抑制作用. 该酶在pH值2.2~8.0保持30 min,酶活保留率都在80%以上,表明此酶耐酸性好,可较好的应用于苹果、柑桔等酸性果汁生产. 经SDS-PAGE和酶谱分析,发现本试验所得PG有聚半乳糖醛酸酶活性的酶活部分为48 kDa左右,有别于已报道的聚半乳糖醛酸酶[27]. 本研究获得的聚半乳糖醛酸酶用于酶解柑桔果胶,通过薄层层析和液相色谱法分析表明可以得到柑桔果胶低聚糖,这为制备功能性果胶低聚糖奠定了基础,为柑桔的综合利用提供了新的的途径.







 DownLoad:
DownLoad: