-
开放科学(资源服务)标识码(OSID):

-
番茄(Solanum lycopersicum)是世界上最主要的蔬菜作物之一. 番茄在生长发育过程中受到了低温、高温、盐碱、水淹、干旱等非生物胁迫的影响,限制其生长、发育甚至最后导致植株的死亡. 热激因子(Heat Shock Factor,HSF)是植物响应高温胁迫的主要调控因子,并参与盐、干旱等胁迫反应过程[1]. 热激因子可分为A,B,C等3类[2-3];其中,A类基因具有转录激活功能[4-5],B类基因起转录抑制作用,B类转录激活因子又可以作为A类转录激活因子的共激活因子,具有辅助激活功能[6-7];而C类基因因为不具有AHA基序,被推测可能不具有转录活性[8-9],但研究发现,C类基因在水稻[10-11]、拟南芥[12-13]、茶[14]、番茄[15-16]等植物中均能受低温显著诱导,是否具有耐寒功能迄今未得到证实. 本研究对番茄SlHsfC1基因在低温、高温、水淹和盐胁迫下的表达特性进行分析,构建该基因的过表达载体,并分析该基因过表达对转基因植株耐寒性的影响,初步证实该基因过表达能增强转基因番茄植株的耐寒性. 该研究结果拟对今后揭示该基因如何参与番茄冷调控的分子机制奠定一定的基础.
HTML
-
供试材料为野生型番茄AC++ (Solanum lycopersicum L. cv. Alisa Craig),由西南大学蔬菜学重点实验室提供.
-
选用的大肠杆菌(Esherichia coli)菌株为DH5α,根癌农杆菌(Agrobacterium tumefaciens)菌株为LBA4404.
-
利用Cluster W软件,对番茄、马铃薯(Solanum tuberosum)、拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、短柄草(Brachypodium distachyon)、莱茵衣藻(Chlamydomonas reinhardtii)、小立碗藓(Physcomitrella patens)的Hsf家族成员的DNA结合域(DNA-banding Domain,DBD)进行多重序列比对,并利用MEGA软件构建上述7种物种DBD结构域的系统发育进化树.
-
选取长势一致“五叶一心”的番茄苗分别放置于4 ℃,37 ℃的人工气候培养箱内,按照正常的光暗周期(16 h光照,8 h黑暗)开始处理,以处理0 h,4 h,8 h,24 h为时间点收取根、茎、叶作为材料,每一个温度处理的各个时间点需保证至少有3株长势一致的番茄苗.
-
选取长势一致的番茄苗,将番茄苗连带营养钵置于深槽托盘中,向深槽托盘中加入定量的水,保证水深在10 cm左右,分别在处理0 d,2 d,4 d,8 d时收集根、茎、叶.
-
选取长势一致的番茄苗,同时放置在含有0,100,200,300,400,500 mmol/L盐水的深槽托盘中,淹没营养钵5 cm左右,处理24 h后收集叶组织.
收集叶片材料时,从下到上选择3~4片真叶. 选取根部材料时,将根的泥土清洗干净,并用滤纸擦干水分,尽快放入液氮中,放入-80 ℃超低温冰箱中保存.
-
采用RNAiso plus(TaKaRa)提取总RNA,CWbio试剂盒(康为世纪)反转录合成cDNA. SlHsfC1基因的qRT-PCR上游和下游引物分别P8和P9. 以番茄中表达稳定的ELF1-α(elongation factor 1-alpha)为内参基因(表 1). 反应染液为Sybergreen(康为世纪),反应体系、表达量计算公式、统计学分析、绘图参照潘阳露[17]的研究方法. 程序为:94 ℃预变性3 min、94 ℃变性30 s、59 ℃退火15 s、40个循环、65~95 ℃解链3 s.
-
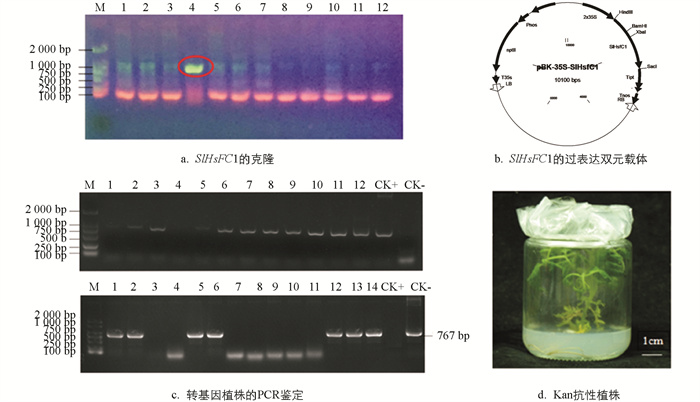
利用巢式PCR(引物见表 1),以番茄cDNA为模板,P2/P3进行第1轮PCR扩增,程序为:98 ℃预变性2 min、98 ℃变性15 s、53 ℃退火15 s、72 ℃延伸80 s、30个循环;以第1轮PCR产物为模板,P4/P5进行第2轮PCR扩增,程序为:98 ℃预变性2 min、98 ℃变性15 s、48 ℃退火15 s、72 ℃延伸80 s、3个循环、98 ℃变性15 s、57 ℃退火15 s、72 ℃延伸80 s、35个循环. 从番茄cDNA中扩增出SlHsfC1的CDS区域,并装入到双元表达载体中. 运用农杆菌介导法转化番茄子叶,具体方法参见潘阳露[17]的研究.
将已生根的卡那霉素(Kan)抗性番茄植株移栽至营养土. 炼苗成活后,取新叶用CTAB法提取gDNA,以nptII基因的引物P6/P7以及目的基因区域定量上游引物P8和终止子Tipt上的引物P1对番茄基因组进行PCR扩增,筛选转基因阳性植株. 以P8/P9引物对进行qRT-PCR分析,鉴别SlHsfC1过表达的转基因番茄植株.
-
将24 ℃培养的过表达转基因阳性植株(T0代)和AC野生型植株经12 ℃冷驯化2 d后,经4 ℃处理7 d后,再经2 ℃处理2 d,观察低温对植株表型的影响. 所有处理的光照条件为16 h光照,8 h黑暗.
1.1. 试验材料
1.2. 菌株
1.3. 生物信息学分析
1.4. 胁迫处理
1.4.1. 低温、高温处理
1.4.2. 水淹处理
1.4.3. 盐胁迫处理
1.5. 定量PCR分析
1.6. 转基因植株的获得
1.7. 转基因植株的耐寒性鉴定
-
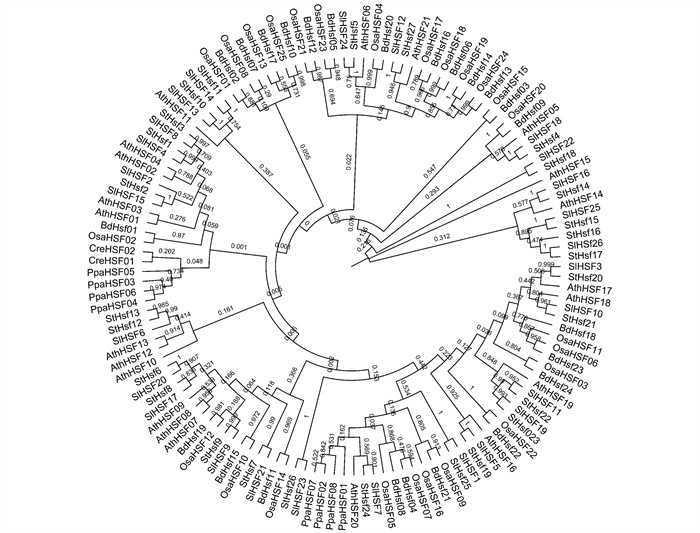
番茄SlHsfC1与其他植物C类热激因子一样,是功能尚不明确的一类转录因子. 在系统进化上,SlHsfC1(SlHsf12)与同科植物马铃薯HsfC1(StHsf27)的亲缘关系最近,两者热激元件的DNA结合域(DNA-banding Domain,DBD)的蛋白序列相似度达到了100%;其次是拟南芥的HsfC1(AthHSF21),再其次是单子叶植物水稻(OsaHSF17,OsaHSF18,OsaHSF19,OsaHSF24)和短柄草(BdHSF06,BdHSF13,BdHSF14,BdHSF16)的HsfC类热激因子;而与A类和B类热激因子的序列相似性相差较大,尤其是在衣藻和苔藓等远古植物种中未发现SlHsfC1的直系同源基因. 此外,在番茄的26个HSF中,SlHsfC1与SlHsf24(SlHsfA3)的序列相似性最大(图 1).
-
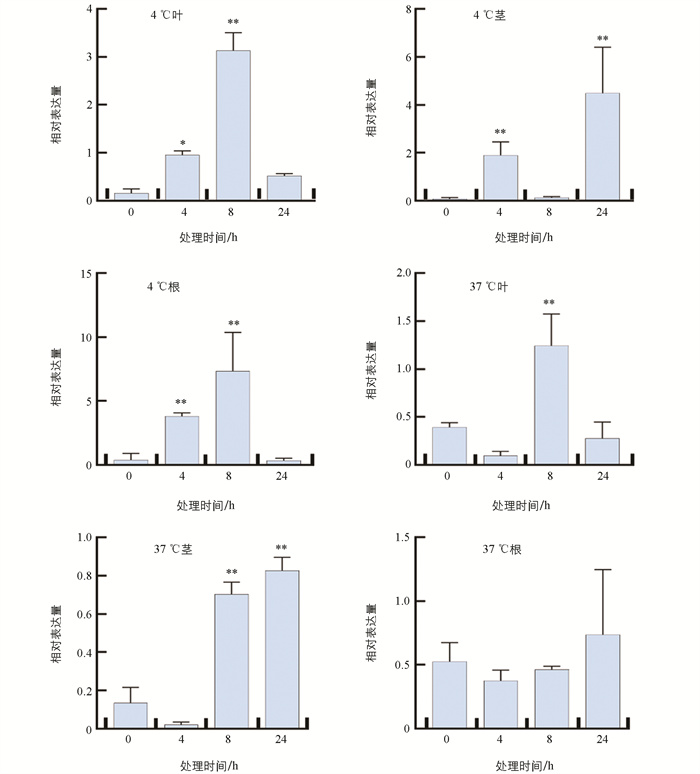
qRT-PCR检查结果显示,低温4 ℃处理4 h,番茄根、茎、叶3个组织中的SlHsfC1的表达量均显著增高;其中,根和叶片在低温处理8 h、茎在低温处理24 h时,与0 h SlHsfC1表达量相比差异有统计学意义(p<0.01). 经过高温37 ℃处理8 h,叶片SlHsfC1的表达量明显增高;高温处理24 h,茎组织SlHsfC1的表达量与0 h相比差异有统计学意义(p<0.01). 表明SlHsfC1能被高温和低温胁迫诱导表达(图 2).
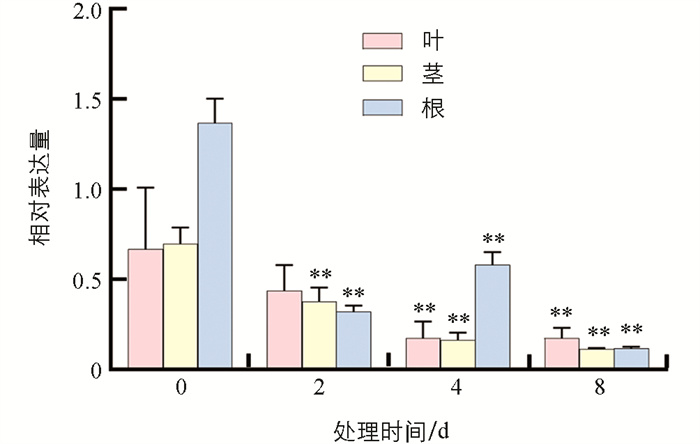
水淹处理后,番茄叶片中SlHsfC1基因表达量在未处理时位于最高值,随着处理时间的延长,SlHsfC1基因表达量逐渐降低,表明SlHsfC1的表达可以被水淹所抑制(图 3).
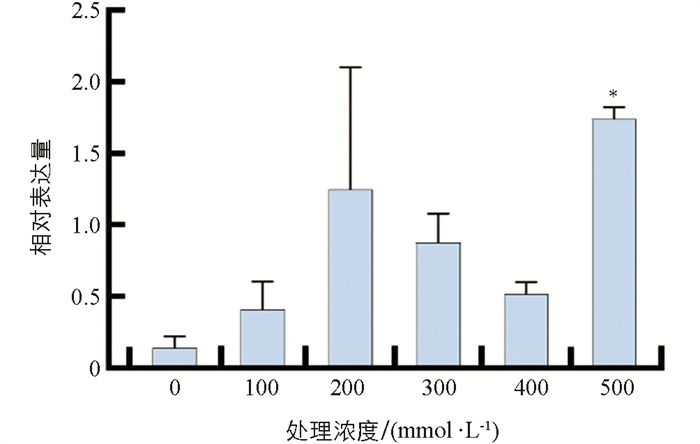
番茄植株经不同浓度的NaCl处理1 d时,SlHsfC1的表达量均表现为升高;其中,500 mmol/L处理下的表达量与对照的差异有统计学意义(p<0.01),表明SlHsfC1可以受盐胁迫诱导表达(图 4).
-
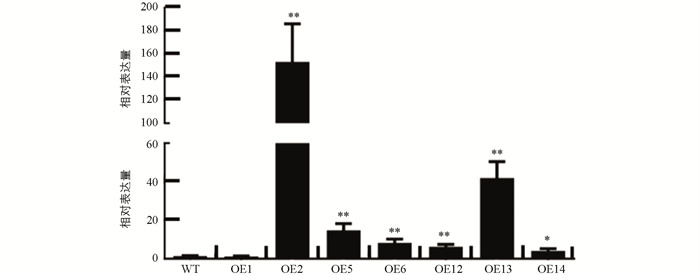
经巢式PCR扩增,得到大小为1 127 bp的SlHsfC1基因CDS全长(图 5a). 构建了该基因的过表达载体(图 5b). 利用农杆菌介导法转化番茄AC的子叶外植体,将SlHsfC1过表达载体的T-DNA整合进番茄AC的基因组中. 经引物对P6/P7、定量上游引物P1对npt Ⅱ和SlHsfC1基因进行PCR扩增,分别得到767 bp和894 bp的目的条带,筛选出SlHsfC1转基因阳性株系10株(图 5c,d). 经qRT-PCR对转基因植株进行检测,鉴别到OE2,OE6,OE12和OE13等SlHsfC1过表达植株. 所有T0代转基因植株的形态与野生型植株没有差别(图 6).
-
为初步鉴别SlHsfC1是否具有耐寒功能,将野生型番茄AC和OE2,OE13过表达植株经过12 ℃,2 d的低温驯化,4 ℃,7 d和2 ℃,4 d的低温处理. 结果发现,野生型番茄AC植株底部叶片完全失水坏死,顶部叶片出现失绿、失水、坏死,而过表达转基因植株OE2,OE13的顶部叶片未出现坏死、失绿,表明SlHsfC1过表达能增强转基因植株的耐寒性(图 7).
2.1. 番茄SlHsfC1与其他HSF蛋白的系统发育关系
2.2. SlHsfC1在低温、高温、水涝和高盐逆境下的表达特性
2.3. SlHsfC1过表达转基因植株的获得
2.4. SlHsfC1过表达能增强转基因植株的耐寒性
-
SlHsfC1在低温、高温、水淹、盐害等非生物胁迫中都有应答,特别是在低温处理后,表达量显著增加. C类热激因子受低温诱导显著表达的现象在拟南芥[10-11]、水稻[10-12]、茶[14]、多毛番茄[15]等植物中普遍存在,因此推测C类热激因子可能参与了冷胁迫调控. 然而,从蛋白序列上分析认为,C类热激因子不具有转录活性[8]. 因此,SlHsfC1等C类热激因子在冷适应中的功能尚不清楚.
本研究发现,SlHsfC1过表达的T0代植株形态正常,但T1代植株明显矮化,与多毛番茄HsfC1基因在普通番茄中过表达和拟南芥HsfC1过表达研究中的矮化现象相一致[15-16]. 由于T1代植株非常矮小,难以进行耐寒性分析,故本研究对T0代长势正常的转基因植株的耐寒性进行了分析,初步证实过表达SlHsfC1能增强转基因番茄植株的耐寒性. 低温反应最重要的CBF(C-REPEAT BINDING FACTORs)过表达后生长受到抑制[17],CBF通过激活GA分解代谢基因GA2ox7促进稳定DELLA蛋白[18-19]. 同时,低温会激活DELLA-GRF的调控,从而抵抗低温胁迫[20]. SlHsfC1是如何参与耐寒性调控,尚待T2代转基因纯合后进行深入的研究.







 DownLoad:
DownLoad: