-
开放科学(资源服务)标识码(OSID):

-
由于水体特殊的理化性质,诸如食物、溶解氧等资源存在巨大的时空异质性,因此生活在水体中的鱼类经常会面临饥饿、低氧等环境胁迫,进而影响其生理功能[1-2].能量代谢是一切有机体正常生理功能得以实现的根本保证.就鱼类而言,静止代谢率(Resting metabolic rate,以RMR表示)和最大代谢率(Maximum metabolic rate,以MMR表示)是其最为重要的能量代谢特征参数,前者反映鱼类维持能量的消耗,后者反映最大有氧功率输出[3-4].此外,运动后过量耗氧(Excess post-exercise oxygen consumption,以EPOC表示)在一定程度上代表鱼类的无氧代谢能力[5-6].已有研究表明,在种内和种间两个水平,鱼类RMR的高低直接决定有氧代谢的输出能力[4, 6-8].鱼类遭受饥饿后,由于生理机能的下调,其RMR和MMR均有不同程度的下降[5, 9],而EPOC对鱼类饥饿的响应尚存争论[5].
低氧耐受是鱼类最为重要的生存适合度特征之一[6, 10].临界氧压(Critical oxygen tension,以Pcrit表示)是指维持鱼类正常RMR的最低溶氧水平,失去平衡点(Loss of equilibrium,以LOE表示)指维持鱼类正常身体姿势的最低溶氧水平,它们常作为鱼类低氧耐受能力的指标[10].已有研究表明,RMR相对较低的个体或物种具有更强的低氧耐受能力[10-11],而因饥饿导致鱼类RMR的下降能否提升其低氧耐受能力的相关研究值得关注.
四川华鳊(Sinibrama taeniatus)俗称墨线鱼,隶属鲤形目、鲤科、华鳊属;主要分布于长江上游岷江、大渡河等水域,是我国长江上游特有的小型经济鱼类[12].该物种偏好激流生境,是食性较为广泛的杂食性鱼类.本研究以四川华鳊为对象,经不同时间(0,3,7,14和28 d)的饥饿处理,测定能量代谢(RMR,MMR和EPOC)和低氧耐受(Pcrit和LOE)特征参数,考察饥饿对其能量代谢和低氧耐受的影响并揭示其生理生态适应对策,旨在为鱼类比较生理学、功能生态学相关领域提供基础资料.
HTML
-
实验用四川华鳊采集于岷江眉山市段,在实验室自净化循环控温水槽中驯养6周,水温为25±0.5 ℃.驯养期间每天以商业饵料(四川斯特佳饲料有限公司)饱足投喂1次,投喂前清除粪便,投喂后1 h左右清除残饵.实验用水为曝气控温后的自来水,并用充气泵不断向驯化水体泵入空气以保证水体溶氧在7.0 mg/L以上,pH值维持在6.5~7.3之间,氨氮保持在0.025 mg/L以内.日换水量约为水体总量的10%,光周期为12 h光照12 h黑暗.驯养完成后,对实验鱼进行不同饥饿时间(0,3,7,14和28 d)的处理.为了消除摄食代谢的影响,对照组(0 d)实验鱼在实验参数测定以前,进行2 d的禁食处理,饥饿组实验鱼分别增加2 d的禁食,其他处理条件与驯养时环境条件保持一致.
-
实验饥饿处理时间完成后,测定实验鱼体质量、体长并计算其肥满度(体质量与体长立方的比值),再将实验鱼转移至呼吸室(容积120 mL),待其适应12 h后,以1 h间隔测定每尾实验鱼耗氧率6次,取平均值作为RMR,采用流水式呼吸仪[13]对实验鱼的耗氧率进行测定.测定完成后,将每尾实验鱼单独放入环形水道并用手进行不断追赶,使其在10 min内力竭[3, 6](有氧和无氧代谢均会涉及);然后立即将力竭状态的实验鱼放回呼吸室,在不同时间点(1~10 min,每1 min测1次;11~60 min,每5 min测1次;61~120 min,每30 min测1次)测定力竭运动后代谢恢复时的耗氧率[3, 6].测定时水温始终保持在25±0.5 ℃,使用溶氧仪(HQ30,Hach Company,Loveland,CO,USA)测定溶氧值,实验鱼耗氧率计算公式为
式中,MO2为耗氧率[mg/(h·kg)],ΔO2为实验呼吸室和对照呼吸室(没有鱼)溶氧的差值(mg/L),v为呼吸室的流量(L/h),m为实验鱼体质量(kg).经计算每分钟呼吸室内水体交换率大于98%,因此不需对恢复期的耗氧率进行相应的校正[14].EPOC(mg/kg)为实验鱼在恢复过程中超过RMR的额外耗氧量,通过极差法对每尾实验鱼的EPOC进行计算.
-
低氧耐受实验鱼RMR测定与EPOC实验鱼RMR测定一致,采用内循环密闭式呼吸仪[15-16]对实验鱼的耗氧率进行测定,进而判定实验鱼的Pcrit和LOE.RMR测定完成后,再将实验鱼转移至低氧耐受测定的呼吸室(容积217 mL),适应1 h后,以3 min的间隔测定呼吸室内的溶氧值直至实验鱼身体失去平衡(侧翻20 s),该点溶氧值为实验鱼的LOE(mg/L)[6, 17].在低氧耐受测定前,呼吸室内水体与外部饱和溶氧水体保持交换(约250 mL/min),以维持呼吸室内正常溶氧水平.测定时水温维持在25±0.5 ℃,实验鱼耗氧率计算公式为
式中,MO2为耗氧率[mg/(h·kg)],ΔO2为相邻两次测定溶氧值的差值(mg/L),V为呼吸室循环系统扣除鱼体积后的体积(L),m为实验鱼的体质量(kg),t为相邻两次测定的间隔时间(3 min,即0.05 h).每尾鱼耗氧率数据收集完成后,采用“双线法”对实验鱼的Pcrit进行判定[18].
-
用Excel 2003对实验数据进行常规计算,用SPSS 17.0软件进行方差分析和回归分析.饥饿时间对代谢和低氧耐受参数的影响,用单因素方差分析;方差分析通过后,各处理组间进行多重比较.在能量代谢(RMR,MMR和EPOC)和低氧耐受(Pcrit和LOE)参数内部以每个处理组的平均值作为一个样本,它们之间的关系用一元线性回归分析.所有数据结果均以x±s表示,当p<0.05时,表明差异有统计学意义.
1.1. 实验鱼的获取与驯养
1.2. 代谢率的测定
1.3. 低氧耐受的测定
1.4. 参数统计与分析
-
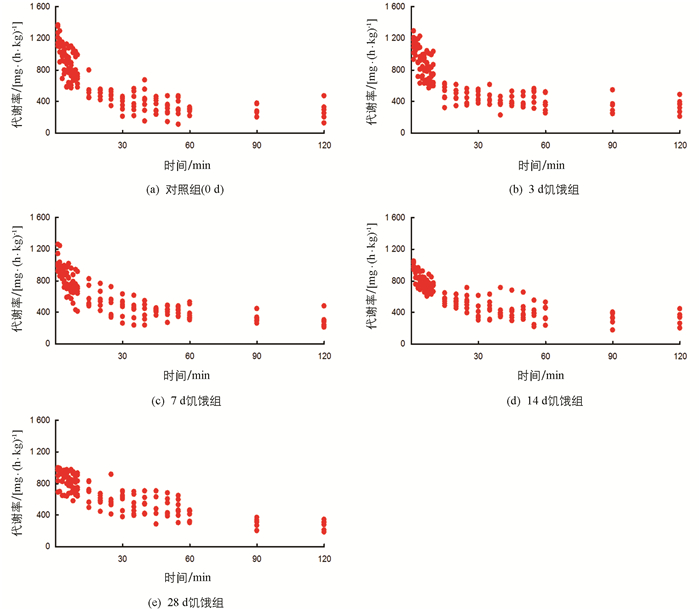
经过力竭运动后,各饥饿处理组实验鱼代谢率均立即增加至最高水平,经恢复后回落至静止代谢率(RMR)水平(图 1),差异有统计学意义(p<0.05).表 1显示,饥饿对实验鱼RMR,最大代谢率(MMR)和运动后过量耗氧(EPOC)均有影响.随饥饿时间延长,实验鱼RMR和MMR均下降,3 d饥饿组RMR与对照组间差异无统计学意义;7,14,28 d饥饿组实验鱼MMR低于对照组,差异有统计学意义(p<0.05).随饥饿时间的延长,实验鱼EPOC增加,14和28 d饥饿组实验鱼EPOC高于对照组,差异有统计学意义(p<0.05).
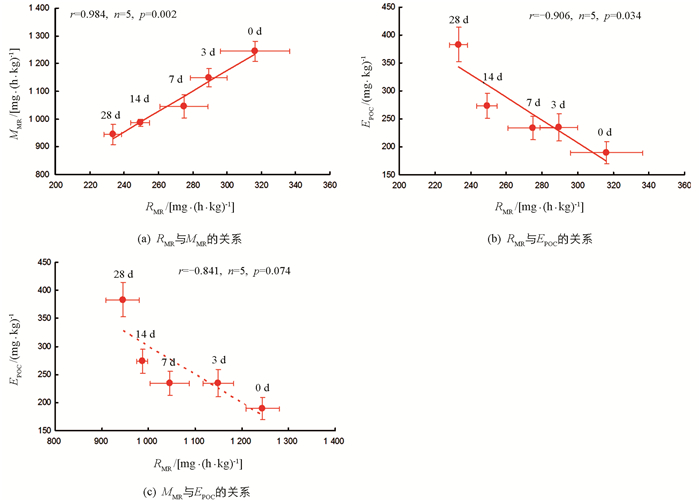
各饥饿处理组,实验鱼RMR与MMR间呈显著正相关(r=0.984,n=5,p=0.002),RMR与EPOC间呈显著负相关(r=-0.906,n=5,p=0.034);MMR与EPOC间相关性无统计学意义(r=-0.841,n=5,p=0.074)(图 2).
-
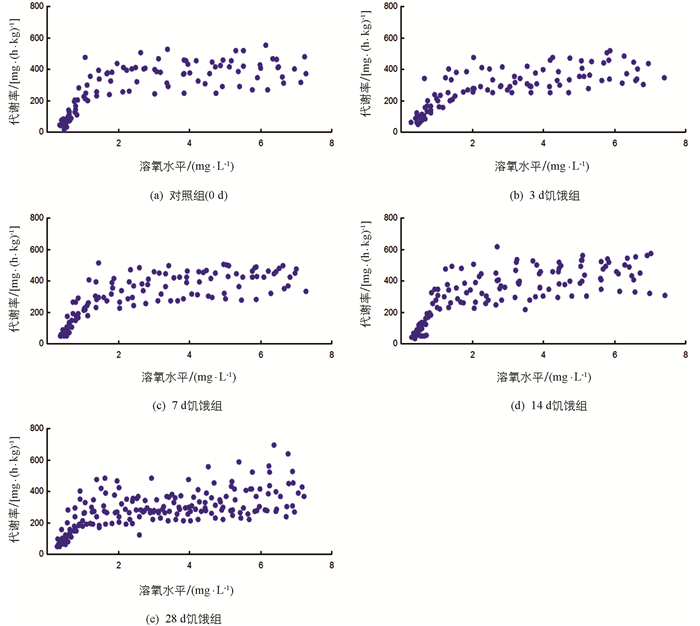
随溶氧水平降低,各饥饿处理组实验鱼代谢率先保持基本不变至临界氧压(Pcrit)水平,溶氧进一步被消耗,实验鱼代谢率下降至失去平衡点(LOE)水平(图 3),下降幅度有统计学意义(p<0.05).表 2显示,饥饿对实验鱼RMR和Pcrit均有影响,部分差异有统计学意义(p<0.05),对LOE影响无统计学意义.随饥饿时间延长,实验鱼RMR和Pcrit均下降.3 d饥饿组RMR与对照组间差异无统计学意义,其余饥饿组RMR低于对照组RMR,差异有统计学意义(p<0.05);28 d饥饿组实验鱼Pcrit低于对照组、3 d和7 d饥饿组,差异有统计学意义(p<0.05).
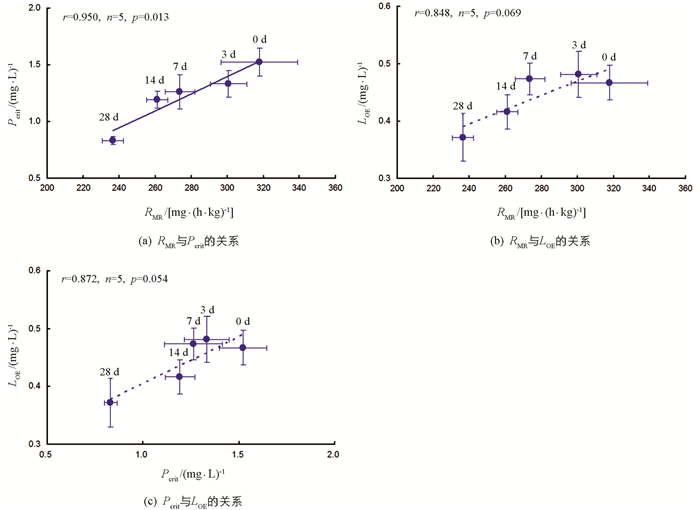
各饥饿处理组,实验鱼RMR与Pcrit间呈显著正相关(r=0.950,n=5,p=0.013);RMR与LOE(r=0.848,n=5,p=0.069)和Pcrit与LOE(r=0.872,n=5,p=0.054)间相关性无统计学意义(图 4).
2.1. 代谢特征
2.2. 低氧耐受
-
鱼类在遭受饥饿时,只能动用身体已贮存的能量物质以维持生命活动,同时下调维持代谢以节约能量的消耗[19].研究发现,四川华鳊在经过7 d饥饿后,其RMR开始下降;经28 d的饥饿后,其RMR下降了26%;与其他鱼类在相同温度条件(25 ℃)、遭受相同时间(28 d)饥饿后相比较,四川华鳊RMR下降比率相对较低.如鲇(Silurus asotu)、南方鲇(Silurus meridionalis)和中华倒刺鲃(Spinibarbus sinensis)遭受饥饿后,其RMR分别下降34%,48%和51%[5, 9, 20].鱼类遭受饥饿后,其生理功能也随之削弱.四川华鳊遭受饥饿后,其MMR也下降,与其他鱼类研究结果类似[5, 9, 20].这种有氧代谢功率输出的下降将削弱鱼类的游泳运动能力[9, 20-21].尽管如此,四川华鳊遭受饥饿后,其EPOC得以提升,与鲇的研究结果相似[5].一方面认为,鱼类遭受非长期饥饿后(4周以内),这种无氧代谢能力的提升是对有氧代谢能力削弱的补偿[5];另一方面认为,饥饿后再进行运动,其生理功能维持状态能量(即RMR)可能会增加,从而导致表观EPOC量的增加[22].
已有研究者认为脊椎动物的最大代谢率与最小代谢率间存在某种必然的关联[7].在自然选择条件下,动物趋向于提高最小代谢率以支持更大有氧运动功率的输出.已有研究发现,无论是种间还是种内水平,动物的最大代谢率与最小代谢率间均呈正相关[6, 8].本研究发现,四川华鳊经不同时间的饥饿(0~28 d),各处理组实验鱼RMR与MMR间呈显著正相关(图 2).鱼类遭受饥饿后,其心鳃呼吸能力、组织结构、相关代谢酶活性均有所下降[23],因此维持能量将会降低,同时伴随最大功率输出的同步下降.该结果与种内水平和种间水平的研究结果一致,表明鱼类RMR的高低是其MMR高低的重要决定因素.
-
研究发现,四川华鳊遭受饥饿后,其Pcrit,LOE均呈下降趋势,表明饥饿后其低氧耐受能力有所增强.结果与以往研究一致,高RMR将有助于提升MMR以实现其相应生理功能,低RMR将会在环境耐受方面获利[4, 6].鲫(Carassius auratus)高RMR个体在遭受饥饿后具有更大的体质量损失率[24].本研究发现,在0~14 d饥饿周期,四川华鳊的肥满度下降24%,而在14~28 d饥饿周期,肥满度仅下降8%,结果表明维持能量的高低与其耐受饥饿能力直接相关.鲤科鱼类不同物种间,RMR与Pcrit间呈正相关,表明低RMR物种具有更强的低氧耐受能力[10].鱼类遭受饥饿后对低氧耐受能力的影响具有复杂的生理过程和机制,一方面由于维持能量的下调(即RMR的降低),对氧的需求减少,可能有助于低氧耐受能力的提升;另一方面,由于饥饿其生理调节(如摄氧、离子耐受等)能力下降,低氧耐受能力将随之被削弱.本研究发现,各饥饿处理组中,RMR与Pcrit间也呈显著正相关(图 4),表明四川华鳊遭受饥饿后由于维持能量的下调,对其低氧耐受能力有一定的补偿作用.经28 d的饥饿处理,四川华鳊Pcrit下降45%,然而LOE仅下降21%,LOE与RMR间相关性无统计学意义(p=0.069).此外,在大西洋鳕鱼(Salmo salar)研究中发现,温度对RMR影响较大,而对LOE影响微弱[25];在30种淡水鱼类的研究中,LOE与RMR之间的关系也不紧密[6].这些结果表明由于RMR的下降,对鱼类LOE的贡献并不明显,因此LOE作为低氧耐受指标具有一定的保守性.
综上所述,四川华鳊在饥饿后,其维持能量消耗降低,从而伴随最大功率输出的下降,而低氧耐受能力有所增强是对某些生理功能(如运动)削弱的补偿,结果显示鱼类应对资源匮乏所采用的生理生态适应对策是经长期自然选择的结果.






 DownLoad:
DownLoad: