-
开放科学(资源服务)标识码(OSID):

-
肉类蛋白是一种高营养价值蛋白,与植物蛋白相比,肉类蛋白具有丰富的必需氨基酸和更高的消化率[1],然而这种高价值蛋白并没有像植物蛋白、牛奶蛋白那样得到充分的利用和开发,部分原因是肉类蛋白中的主要蛋白——肌原纤维蛋白在低离子介质或等电点附近具有较差的功能特性(分散、乳化、凝胶)[2-3]. 在低离子介质中肌原纤维蛋白中的最主要蛋白——肌球蛋白容易通过尾部静电吸引缠绕自组装形成螺旋结构,导致蛋白聚集、沉降,致使蛋白功能特性下降[4]. 在等电点附近,蛋白分子间由于电荷屏蔽效应也会引起肌球蛋白自组装及聚集[5],因此,调节分子间的电荷作用能一定程度改善肌原纤维蛋白在低离子介质或等电点附近的功能特性.
卡拉胶是一种硫酸化阴离子多糖,这种亲水胶体分子侧链带有大量负电荷,当它与蛋白分子形成络合物后,多糖提供的电荷能改善蛋白的功能特性[6-7]. 此外,多糖分子自身的空间位阻和黏性也能在一定程度上阻碍蛋白的聚集,改善蛋白的功能,例如蛋白的溶解能力[8]等. 卡拉胶也常被作为一种稳定剂用来控制乳品流体的质地特性[9],广泛用于控制乳清蛋白[10]、牛奶蛋白[11]、大豆分离蛋白[12]乳液流体品质,然而卡拉胶如何影响肉类蛋白的乳化能力还鲜有报道. 此外,流体食品的开发、设计过程中往往要考虑消费者的适口性,而微酸的蛋白饮料(pH值为4.0~6.0)具有较好的口感和风味[13]. 对于肌原纤维蛋白来说,其等电点在pH值为5.5附近,此时蛋白因电荷的减少而导致功能特性受到负面影响,因此,本研究以促进肌原纤维蛋白在低离子介质和等电点附近分散能力为目的,通过超声改性以及多糖混合,进一步提高蛋白在微酸条件下的分散和乳化能力,为肉类蛋白的进一步开发流态食品提供理论依据.
HTML
-
实验所用兔肉样本来自重庆阿兴记兔场,均为雄性伊拉兔(2.3~2.5 kg,75日龄). 按照商业屠杀标准,去皮和内脏后将胴体放入6 ℃冰箱,1 h内运往实验室,并贮藏于-20 ℃冰箱中冷冻保存. 卡拉胶(纯度99%),法国Louis Francois公司;磷酸氢二钠、磷酸二氢钠、乙二胺四酸钠、酒石酸钾钠、五水硫酸铜、氢氧化钠、盐酸、氯化钠、氯化镁、十二烷基硫酸钠均为分析纯,重庆跃翔科技有限公司;大豆油,益海嘉里金龙鱼粮油食品股份有限公司.
-
绞肉机(MC-30X1-101A1),美的集团;匀浆均质机、冷冻干燥机,宁波新芝生物科技股份有限公司;pH计,上海仪电科学仪器股份有限公司;台式高速离心机(5810型),德国Eppendorf公司;冷冻离心机,美国贝克曼库尔特公司;光学显微镜,日本奥林巴斯株式会社;紫外-可见分光光度计,日本岛津仪器有限公司;Zeta电位仪,英国马尔文公司;磁力搅拌器,德国艾卡;超声波细胞破碎仪,南京舜玛仪器设备有限公司.
-
将兔后腿肉在4 ℃下解冻12 h,并将解冻的兔肉用绞肉机搅成肉泥状. 将25 g肉泥与100 mL的磷酸盐(PBS)缓冲液混合(20 mmol/L中含100 mmol/L NaCl,2 mmol/L MgCl2,1 mmol/L EDTA-2Na);然后在8 000 r/min的条件下均质1 min,间歇1 min,再均质1 min;匀浆后,将肉糜在5 000 r/min,4 ℃条件下离心15 min,并移除上清液;随后将100 mL PBS缓冲液再次加入肉糜沉淀重复均质、离心操作2次;去除上清液后,100 mL PBS缓冲液(20 mmol/L,pH值为6.25,100 mmol/L NaCl)加入并按照相同的参数均质、离心,最后去除上清液,向沉淀中加入100 mL超纯水(Milli-Q). 混合物按上述条件进行2次均质、离心、去除上清液操作,再用4层纱布过滤. 将样品溶解于超纯水中,并调节其浓度为10 mg/mL.
-
将超声探头浸入蛋白样品溶液距底部1.5 cm处,使用外加冷却装置使分散液温度保持在(4±0.5) ℃. 超声波细胞破碎仪(SM-1000A)采用间歇工作状态(2 s打开,3 s关闭),超声频率为25 kHz,功率为600 W处理15 min,处理结束后蛋白样品作为对照. 将对照蛋白溶液分别用0.6 mol/L盐酸调节pH值至6.5,6.0,5.5,5.0,4.0,在测定蛋白溶解度之前将样品放置4 ℃冰箱保存. 独立处理3批样品.
-
将蛋白分散液浓度稀释至4 mg/mL,然后在10 000 r/min的条件下离心10 min,取蛋白上清液并用双缩脲试剂测定其浓度. 蛋白溶解度(S)按公式计算:
式中:P上清为上清液蛋白浓度;P总为总蛋白浓度.
-
卡拉胶-肌原纤维蛋白络合物构建参照Chen等[3]的研究稍作修改,蛋白浓度为10 mg/mL,卡拉胶与蛋白按照3∶7的体积比混合,使得最终混合体系中蛋白与卡拉胶的质量比分别为8∶1,4∶1,2∶1. 用磁力搅拌器将蛋白与卡拉胶混合均匀,同时用0.6 mol/L盐酸溶液调节混合液pH至蛋白等电点附近(5.0,5.5,6.0),该过程独立处理3批样品.
-
将蛋白分散液分别用对应pH值的盐酸-氢氧化钠溶液稀释至0.5 mg/mL,将稀释液进行Zeta电位分析. 测试过程采用SOD模式,平衡时间为120 s,测试温度为25 ℃.
-
蛋白乳化能力和稳定性的测定与计算参照Agyare等[14]的研究,将20 mL蛋白溶液和5 mL大豆油加入50 mL塑料离心管中,并在10 000 r/min的条件下均质1 min,然后取10 mL溶液加入平底顶空瓶中并开始计时,在0 min和10 min时从瓶底(约0.5 cm处)取出50 μL的样品分散于5 mL 0.1% SDS溶液中.
-
利用光学显微镜观测蛋白乳液的形态,分别取不同处理组50 μL乳液样品转移到载玻片上,然后用盖玻片覆盖,在室温下用400倍放大镜进行观测.
-
将不同处理组的蛋白冷冻干燥,并研磨成细粉末用于红外光谱分析,光谱范围为400~4 000 cm-1,使用Origin 8.1软件确定区间峰的位置.
-
实验独立处理3批样品,每次重复测量3次. 数据采用SPSS 22软件进行处理,采用方差分析(ANOVA) 对结果的差异显著性进行评价,p<0.05为差异有统计学意义,图形用Origin 8.1进行绘制.
1.1. 材料和试剂
1.2. 仪器和设备
1.3. 实验方法
1.3.1. 兔肉肌原纤维蛋白的提取
1.3.2. 超声处理中性pH条件下肌原纤维蛋白分散液
1.3.3. 蛋白溶解度测定
1.3.4. 卡拉胶-肌原纤维蛋白络合物构建
1.3.5. 等电点附近蛋白Zeta电位分析
1.3.6. 等电点附近蛋白乳化能力测试
1.3.7. 乳液形态观察
1.3.8. 红外测定
1.3.9. 数据处理
-
肌原纤维蛋白是一种盐溶性蛋白,在低离子强度介质条件下由于有序的大分子肌纤维结构以及肌球蛋白有规律的自组装过程,导致其容易聚集、沉降,分散能力较差[4]. 图 1中,当肌原纤维蛋白未经过超声处理时,蛋白溶解度较低(23.51%)且分散能力较差;当蛋白经过高强度超声处理后,其溶解度显著上升,最高达到77.32%(p<0.05). 超声空化效应带来的强烈物理作用力,包括剪切、冲击、湍流会导致肌原纤维蛋白发生去折叠效应,促进丝状肌球蛋白结构的伸展及随机解离,导致蛋白溶解度显著增加,分散能力增强[15]. 为了考察超声处理后的肌原纤维蛋白在微酸条件下的分散能力,进一步探究其在不同pH条件下的溶解特性,结果发现当介质pH越接近蛋白等电点时,蛋白溶解度显著下降(p<0.05),在pH值为5.5时达到最低(8.25%);当pH值进一步下降至4.0时,其溶解度显著增加(p<0.05). 由于静电屏蔽效应,等电点附近的蛋白分子表面静电荷最低,此时分子间具有最小的静电斥力. 在pH值为5.5时,由于蛋白处于等电点附近,肌球蛋白此时更容易组装成丝状聚集物,蛋白溶解度下降[5, 16]. 当pH远离等电点时,分子间的静电斥力随着静电荷的增加而增加,促进了肌纤维丝之间的解离,同时创造了更多的结合位点,例如与水形成的氢键或介质中形成的盐离子键[5],导致溶解度显著提升.
-
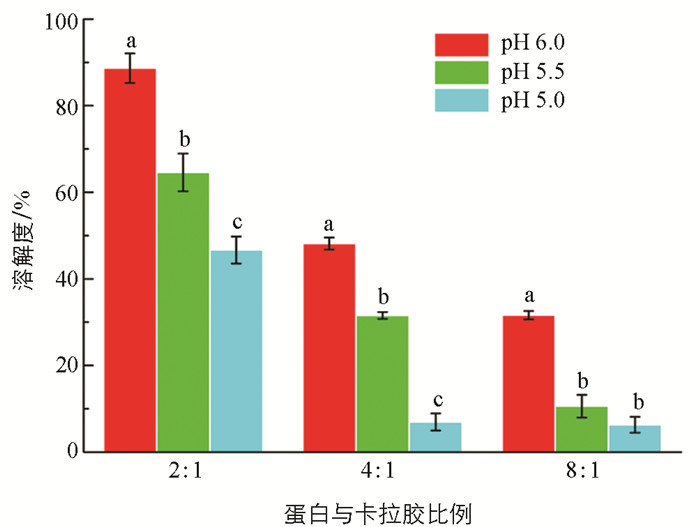
溶解度是蛋白质的一个重要功能指标,它决定着蛋白流体的分散能力,对蛋白的其他功能特性如凝胶、乳化能力也有显著的影响. 表面电荷的缺失导致蛋白过度聚集,乳化能力下降[17]. 蛋白质与天然或合成的聚电解质在室温下的络合作用会显著影响复合物内蛋白质的聚集状态,因为络合物内蛋白质的净电荷以及构象和疏水性都会发生变化[18-19]. 为了进一步改善肌原纤维蛋白在等电点附近(pH值为5.0~6.0)的分散能力,通过添加卡拉胶以构建蛋白-多糖络合物来改善其在等电点附近的溶解度,其结果如图 2. 随着卡拉胶含量的增加,在等电点附近蛋白的溶解度得到显著改善(p<0.05). 卡拉胶作为一种常见的阴离子多糖,其侧链含有大量的负电荷,当其与蛋白形成复合物会为蛋白表面提供更多的负电荷,促进蛋白分子的解离,结果与Klemmer等[20]的研究类似. 当豌豆分离蛋白与海藻酸钠形成复合物时,高浓度的海藻酸钠(0.5%)会提供大量的表面电荷,致使蛋白分子间静电斥力增加,阻碍分子间的相互作用和聚集,因此蛋白浊度降低. Lan等[21]也发现高甲氧基果胶浓度的增加会导致豌豆分离蛋白在等电点附近的溶解度显著提升(p<0.05). Chen等[3]发现果胶浓度增加会显著降低等电点附近肌原纤维蛋白的浊度,抑制蛋白的聚集. 一般来说,阴离子多糖与蛋白质带正电荷的侧链(如氨基)在中性pH条件下通过静电相互作用形成络合物[22],导致蛋白在等电点附近的表面净电荷以及构象和疏水性发生变化.
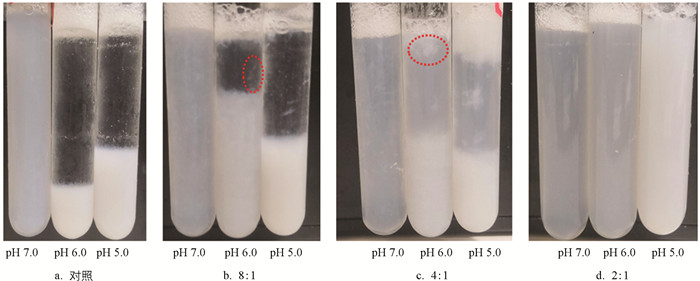
溶解度是反应蛋白流体稳定性的一个最重要指标. 为了进一步证实卡拉胶能改善蛋白在等电点附近流体稳定性的能力,对络合蛋白在4 ℃条件下贮藏7 d后对流体稳定性进行观察,结果如图 3. 当蛋白未添加卡拉胶时,由于超声的物理修饰,在中性低离子强度条件下(pH值为7.0),蛋白呈均匀分散状态. 随着pH值逐步接近于蛋白等电点,分散液逐渐开始出现聚集、沉降,这个现象对应了蛋白溶解度的结果. Liu等[15]的研究结果发现,超声处理(20 kHz,450 W)15 min能导致肌原纤维蛋白内部完整的丝状肌球蛋白结构解离,阻碍其组装成丝. 此外,超声的空化效应也会促进蛋白去折叠效应并暴露出内部疏水基团,蛋白通过二硫键交联形成可溶性低聚物,提高蛋白的流体稳定性. 等电点附近表面电荷的减少使得蛋白迅速聚集、沉降,当添加卡拉胶后,蛋白的流体稳定性逐步提升,当蛋白与卡拉胶比值达到4∶1时,仍然有相分层的现象发生. 此外,蛋白和卡拉胶比值在8∶1和4∶1时,蛋白上层液面还能观测到部分的多糖-蛋白絮凝物的产生,这可能是由于较低的胶体浓度所携带的电荷不足以促使蛋白分散,同时卡拉胶与蛋白形成了更大分子量的络合物所致. 当蛋白与卡拉胶比值为2∶1时,在等电点附近蛋白处于完全分散的状态,而pH值为5.0时具有更高的蛋白浊度,这可能是静电荷的进一步减少导致蛋白与卡拉胶形成了络合物.
-
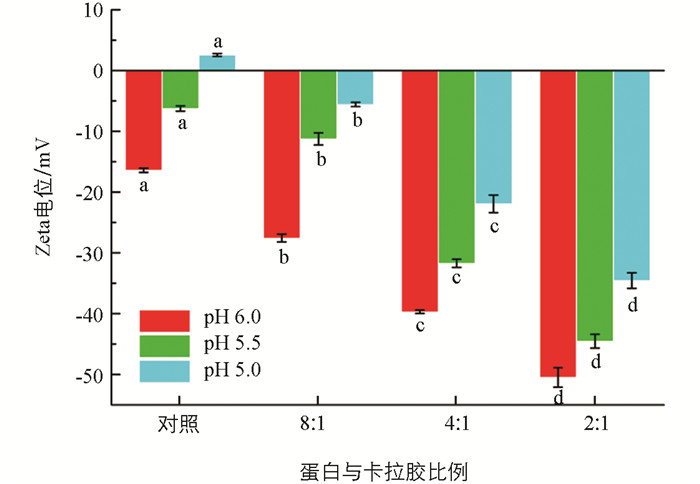
Zeta电位值能反应蛋白表面电荷大小,与蛋白的分散与溶解密切相关. 为了进一步证实卡拉胶-蛋白络合后蛋白的流体稳定与表面电荷有关,对蛋白的Zeta电位进行进一步分析,结果如图 4. 当未添加卡拉胶时,介质pH越接近等电点,蛋白的表面电荷绝对值逐渐降低,从|-16.64| mV下降至|-6.24| mV. 随着pH值进一步下降至5.0,分子表面带正电荷(2.58 mV). 蛋白的Zeta电位绝对值随着pH值的下降逐渐降低,对应了溶解度的下降(图 1),表明表面电荷显著影响了蛋白的分散和流体稳定. 分子表面电荷变化与蛋白的侧链氨基与羧基团密切相关,当pH值低于等电点时,由于质子化胺(NH3+)的存在电荷为正;当pH值高于等电点时,由于去质子化羧基存在(COO-),蛋白表面带负电. 随着卡拉胶浓度的增加,蛋白的表面电荷显著增加(p<0.05). 这个结果也与Ortiz等[23]的研究结果类似,他们发现当介质pH值逐步朝着酸性条件偏移时,大豆蛋白的溶解度逐渐降低.
-
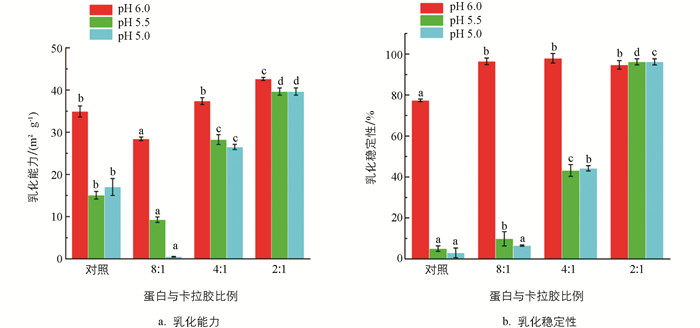
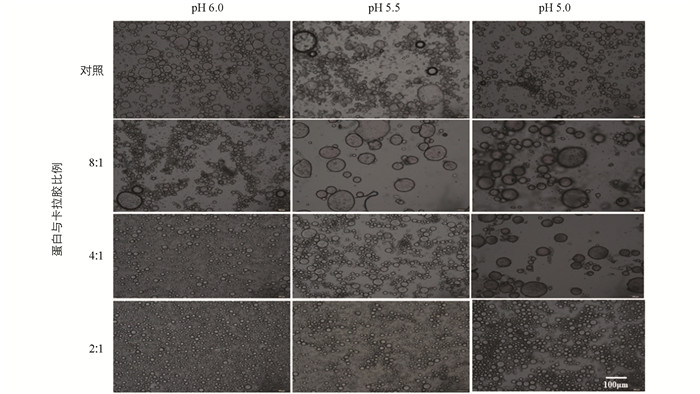
流体食品通常以乳液的形式存在(如O-W),蛋白的两亲性(亲/疏水性)也通过降低界面张力促进其快速扩散和/或吸附到油水界面[24-25],然而,当介质pH接近蛋白等电点附近时,由于电荷的缺失导致蛋白聚集,包裹油滴能力下降. 阴离子多糖具有良好的凝胶、增稠和稳定的特性,是制备多层乳液最常见的聚电解质[26]. 这些吸附在多层乳液最外层的阴离子多糖通常具有较强的空间位阻和静电排斥作用,可以大大提高多层乳液的物理稳定性. 图 5结果显示,当卡拉胶浓度较低时,蛋白在等电点附近的乳化能力较差. 乳化能力值与蛋白质在乳化过程中迅速吸附到水-油界面以防止聚结和絮凝的能力有关. Mcclements等[27]发现,当多糖浓度较低时,体系中的多糖浓度不足以完全饱和油滴表面,此时一个多糖分子将由多个油滴共享,乳液就会产生絮凝. 虽然少量的卡拉胶(蛋白与卡拉胶比例为8∶1)提高了络合物的表面电荷,但是较低浓度的卡拉胺导致与蛋白分子不能完全络合,形成了更大程度的分子聚集. 为了证实乳液的絮凝、聚集现象,利用光学显微镜对乳液形态进行了观察(图 6),在pH值为5.5时,少量多糖(8∶1)与对照相比乳液有更大程度的絮凝和聚集. 结果进一步证实了少量多糖会进一步促进蛋白聚集,导致乳液絮凝. 随着多糖浓度的逐渐提升,乳液体系趋于稳定,油滴开始逐步呈现均匀、分散状态,蛋白的乳化能力和乳化稳定性均显著提高(图 5).
-
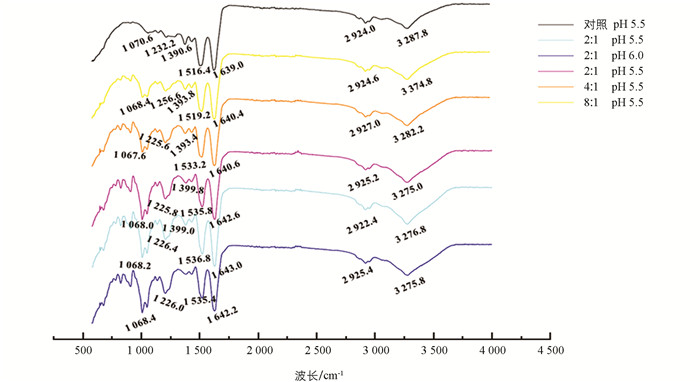
为了进一步明确肌原纤维蛋白与卡拉胶在等电点附近的作用机制,利用红外光谱剖析卡拉胶与蛋白络合对蛋白内部分子键的影响,结果如图 7. 蛋白分子间键的变化在红外区域能够完全反映,例如在1 600~1 700 cm-1为酰胺Ⅰ带波段反映C=O拉伸;1 500~1 600 cm-1为酰胺Ⅱ带波段反应N-H振动,而1 200~1 350 cm-1为酰胺Ⅲ带波段反应C-N拉伸;3 400 cm-1为酰胺A带波段反映N-H拉伸. 这些区间形成峰的强度或偏移能反应蛋白结构和内部分子键的变化[28]. 在pH值为5.5时,与对照相比,添加卡拉胶导致蛋白的酰胺A带发生明显的蓝移,表明蛋白内部氢键发生了明显的改变[29]. 酰胺A带峰的偏移也与分子羟基(-OH)振动伸缩有关. 卡拉胶作为一种亲水性胶体,带来的亲水性羟基(-OH)一定程度上能够改善蛋白的分散能力,因此提高了其在等电点附近的溶解度(图 2). 此外,与卡拉胶络合后蛋白在酰胺Ⅰ带和Ⅱ带的峰发生了明显的偏移(图 7),这不仅表明多糖的羧基(-COO)与蛋白分子的氨基(NH3+)发生了静电相互作用,也与蛋白新形成的肽键拉伸(CO-NH)有一定关联[30]. 卡拉胶与蛋白络合后,较大分子量带来的空间位阻及表面电荷能够促进蛋白分子发生解螺旋效应或结构重排[31],促进肌原纤维蛋白在等电点附近的解离,阻碍肌球蛋白自组装过程. 解螺旋效应也伴随着内部疏水基团的进一步暴露而提高了络合后蛋白的两亲性,因此蛋白在等电点附近的乳化能力得到进一步提升.
2.1. pH对超声处理肌原纤维蛋白溶解度的影响
2.2. 卡拉胶对等电点附近超声处理后肌原纤维蛋白溶解度和流体稳定性的影响
2.3. 卡拉胶对等电点附近超声处理后肌原纤维蛋白Zeta电位的影响
2.4. 卡拉胶对等电点附近超声处理肌原纤维蛋白乳化能力的影响
2.5. 红外分析卡拉胶与蛋白络合对蛋白内部分子键的影响
-
超声处理能够显著改善兔肉肌原纤维蛋白在中性pH、低离子介质中的分散能力,在等电点附近由于表面电荷减少,分散能力显著下降. 卡拉胶与蛋白络合后会提高蛋白的表面电荷,破坏分子氢键,促进蛋白分子发生解螺旋效应,从而暴露内部的疏水基团,提高蛋白的两亲性,因此显著改善了蛋白在等电点附近的分散及乳化能力. 这种变化受到卡拉胶添加量的影响,在等电点附近(pH值为5.5),较低的卡拉胶浓度(蛋白与卡拉胶比例为8∶1)会与蛋白形成大分子量的络合物,降低了蛋白的乳化能力;随着卡拉胶浓度的升高,蛋白在等电点附近的乳化能力显著改善. 该研究为兔肉肌原纤维蛋白流态食品的设计和开发提供了一定的理论基础.







 DownLoad:
DownLoad: