-
开放科学(资源服务)标识码(OSID):

-
羊肚菌(Morchella esculenta)隶属子囊菌门盘菌目羊肚菌属,是一种珍稀药食兼用菌,味道鲜美、风味独特[1-2]. 研究发现,羊肚菌富含多种生物活性成分,如多糖、蛋白质、微量元素、氨基酸等[3-4],具有抗氧化[5]、抗炎[6]、抗肿瘤[7]、抗癌[8]、抗糖尿病[9]、降胆固醇[10]、免疫调节[1]等功效. 生鲜羊肚菌代谢旺盛,菌盖脆嫩,采摘和运输过程易受机械损伤,常温放置易水分散失,子实体褐变、软烂、发霉及腐臭[11]. 化学保鲜剂[12]、乳酸链球菌素[13]、棘托竹荪菌丝体保鲜液[14]、壳聚糖保鲜液[15]、微酸性电解水[16]等保鲜通常采用浸渍处理,易造成子实体相对湿度过大,不利于贮运保鲜;高压电场[17]、辐照[18-19]保鲜等对设备要求较高. 被动自发气调贮藏(PMAP)[20]因保鲜膜种类、特性繁多,难以制定针对特定食用菌保鲜统一的包装标准[21]. 主动自发气调贮藏(AMAP)[22]因充入特定气体成分,可快速达到所需气体环境实现保鲜[23],在物流保鲜中具有独特优势,但需要气调设备支撑. 随着消费健康和食品安全意识的不断提高,探寻不影响生鲜食用菌感官品质和营养价值,同时又易操作实施、成本低廉、绿色安全的配送保鲜方案成为业内首选. 臭氧和紫外处理,具有操作方便、设备投入少等优点,不同浓度臭氧对羊肚菌的保鲜作用[24]已有报道;紫外处理也已见白玉菇[25]、双孢菇[26]、香菇[27]、鸡腿菇[28]等食用菌保鲜,但针对羊肚菌紫外处理未见有单独报道. 本文以重庆黔江主栽羊肚菌品种“七妹”为试材,研究UVC不同处理时间对其感官、褐变度、蛋白质、可溶性固形物、相关抗性酶SOD、CAT活性及次生代谢产物MDA含量的影响,探究适宜的UVC处理时间,以期为生鲜羊肚菌紫外保鲜配送提供理论依据.
HTML
-
供试羊肚菌取自重庆市黔江区重庆市璞琢农业开发有限责任公司,新鲜采摘.
考马斯亮蓝G-250,国药集团化学试剂有限公司;超氧化物歧化酶(SOD)试剂盒,丙二醛(MDA)含量检测试剂盒,北京索莱宝科技有限公司.
-
飞利浦TVU30W紫外线消毒灯,北京金城合作科贸有限公司;754E紫外—可见分光光度计,天津市普瑞斯仪器有限公司;GL-12A高速冷冻离心机,上海菲恰尔分析仪器有限公司.
-
试验设3个处理,将采摘的生鲜羊肚菌剪平菌脚,随机分为3组,每组500 g,分别采用功率30 W、波长254 nm的紫外灯于超净工作台上照射20 min,30 min,40 min,然后单层摆放,保湿纸隔离放置于泡沫箱中,(4±0.5) ℃控温贮藏,每隔2 d测定感官品质、褐变度、可溶性固形物、可溶性蛋白、SOD、CAT及次生代谢产物MDA等指标,重复3次.
-
由经过培训的10人组成评价小组,分别从腐烂、质地、褐变、味道4个方面对贮运过程生鲜羊肚菌品质进行感官评定(表 1),取4项得分总和作为结果.
-
褐变度参照单楠等[29]的方法,精准称取3.00 g羊肚菌,冰浴研磨转移至25 mL容量瓶中,并用0.2 mol/L磷酸盐缓冲液(pH值为6.5)定容至刻度,混匀提取10 min,然后5 000 r/min离心10 min,取上清液,在450 nm波长处测定吸光度(A450),按公式计算样品褐变度(B):
-
手持式折光仪,参照曹建康等[30]的方法. 随机取3~5个羊肚菌子实体,放入研钵捣碎,用干净纱布榨汁,用小滴管取少量汁液滴在折射仪测试玻璃上进行测定.
-
考马斯亮蓝染色法,取0.3 g羊肚菌样品,加入蒸馏水或缓冲液研磨成匀浆后,于4 ℃,12 000 r/min离心20 min,收集上清液即为可溶性蛋白提取液,低温保存备用.
吸取0.5 mL样品提取上清液(视蛋白质量适当稀释),放入具塞试管中,加入5.0 mL考马斯亮蓝G-250溶液,充分混合,放置2 min后在波长595 nm处比色,按照制作标准曲线同样的方法测定吸光度值,重复3次,按公式计算可溶性蛋白质量分数(C):
式中:m′为从标准曲线查的蛋白质量(μg);v为样品提取液总体积(mL);Vs为测定时所取样品提取液体积(mL);m为样品质量(g).
-
参照超氧化物歧化酶(SOD)试剂盒说明书进行,以每分钟每克样品在反应体系中使560 nm处吸光值变化0.01为1个酶活力单位(U/g).
-
参照曹建康等[30]的方法,取0.2 g羊肚菌样品置于研钵中,加入5.0 mL提取缓冲液,在冰浴条件下研磨成匀浆,于4 ℃,12 000 r/min离心30 min,收集上清液即为酶提取液,低温保存备用.
酶促反应体系由2.9 mL 20 mmol/L H2O2溶液和100 μL酶提取液组成,以蒸馏水为参比空白,在反应15 s时开始记录反应体系在波长240 nm处的吸光度值作为初始值,然后每隔30 s记录1次,连续测定,至少获取6个点的数据,重复3次.
式中:ΔOD240为每分钟反应混合物吸光度的变化值;OD240F为反应混合物吸光度终止值;OD240I为反应混合物吸光度初始值;tF为反应终止时间(min);tI为反应初始时间(min).
以每克样品(鲜质量)每分钟吸光度变化值减少0.01为1个过氧化氢酶活性单位,计算公式:
式中:U为过氧化氢酶活性(U/g);v为样品提取液总体积(mL);Vs为测定时所取样品提取液体积(mL);m为样品质量(g).
-
参照丙二醛(MDA)测试盒说明书进行,单位为nmol/g.
1.1. 材料与试剂
1.2. 仪器与设备
1.3. 试验设计
1.4. 测定指标与方法
1.4.1. 感官品质评价
1.4.2. 褐变度
1.4.3. 可溶性固形物
1.4.4. 可溶性蛋白
1.4.5. 超氧化物歧化酶(SOD)活性测定
1.4.6. 过氧化氢酶(CAT)活性测定
1.4.7. 丙二醛(MDA)含量测定
-
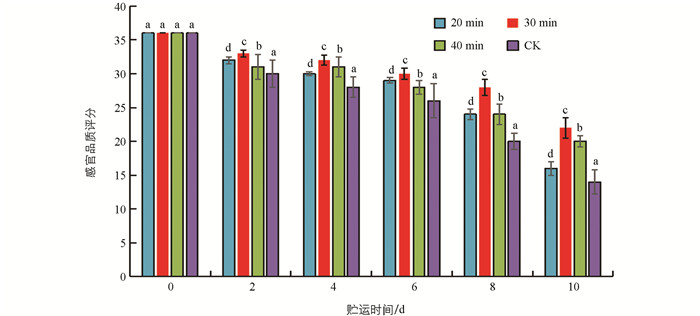
生鲜羊肚菌贮运过程易发生菌柄褐变、菌盖萎蔫软烂、菌盖褶皱脱落以及内容物外溢等品质劣变现象. 图 1显示,随着贮运时间的延续,生鲜羊肚菌感官评分呈下降趋势,30 min UVC处理2 d,4 d,6 d,8 d,10 d其感官评分分别比CK对照提高10.15%,14.29%,15.38%,40.05%和57.14%,优于20 min和40 min. 40 min UVC处理不及30 min,可能与紫外处理过度伤害有关.
-
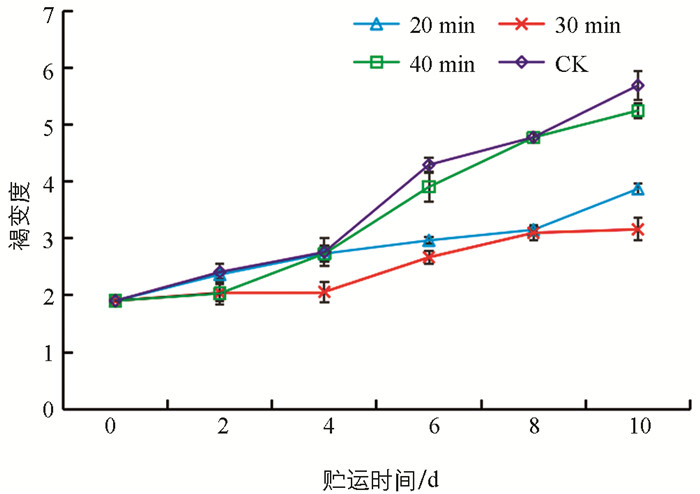
食用菌含有大量酚类物质,生鲜食用菌采后仍维持活跃的呼吸代谢活动. 氧的大量侵入,使得多酚类物质被氧化,造成醌类物质大量形成,醌类物质又大量聚合成黑色素,从而发生褐变. 图 2显示,生鲜羊肚菌褐变度随贮运时间的延长呈上升趋势,UVC处理对褐变发生有一定的抑制作用,30 min UVC处理2 d,4 d,6 d,8 d,10 d分别比CK对照减少15.21%,25.33%,37.86%,35.17%和44.46%,优于20 min和40 min. 20 min,30 min UVC处理与CK对照相比,可明显抑制褐变发生(p<0.05),而40 min可能对其造成紫外伤害,加剧了褐变反应,效果与CK对照相当.
-
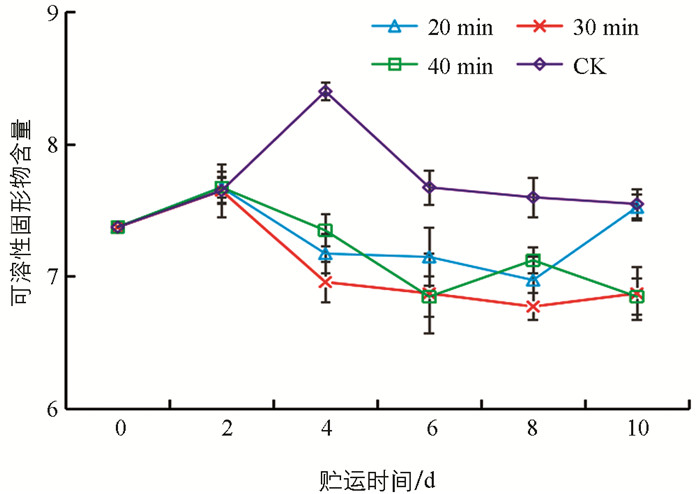
可溶性固形物含量与果蔬成熟程度有关[31],贮藏保鲜的目的之一就是延缓可溶性固形物含量上升的速度[32],可作为一个反映羊肚菌贮藏品质变化状况的指标. 图 3显示,贮藏前期可溶性固形物呈上升趋势,这与羊肚菌后熟有关;贮藏中后期可溶性固形物呈降低趋势,这与老化有关. UVC处理在贮藏中后期可溶性固形物均低于CK对照,表明UVC处理起到了抑制羊肚菌成熟老化的作用. 30 min UVC处理可溶性固形物含量在整个贮藏期间处于最低水平,说明该处理可以有效降低羊肚菌可溶性固形物含量的上升速度,与于晋泽等[24]使用臭氧保鲜羊肚菌的结果一致.
-
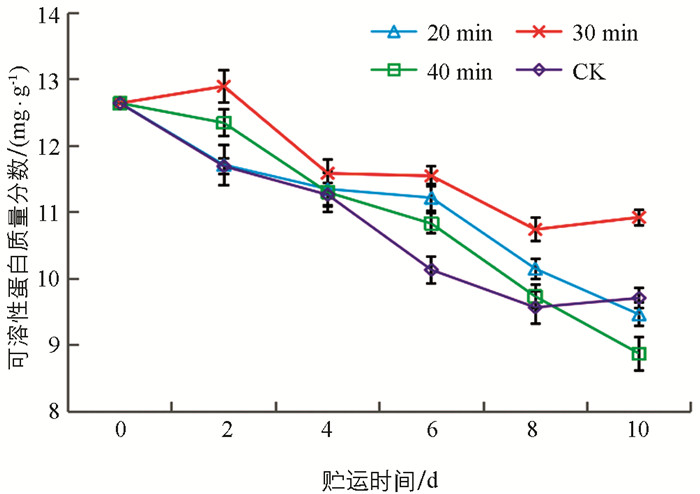
可溶性蛋白是重要的渗透调节物质和营养物质,其增加或积累能提高细胞的保水能力,对细胞生命物质及生物膜起保护作用. 图 4显示,UVC处理延缓了可溶性蛋白质量分数的下降趋势,30 min UVC处理2 d,4 d,6 d,8 d,10 d分别比CK对照增加10.29%,2.87%,13.96%,12.26%和12.51%,优于20 min和40 minUVC处理,保持相对较高的可溶性蛋白质量分数,有利于生鲜羊肚菌的保鲜.
-
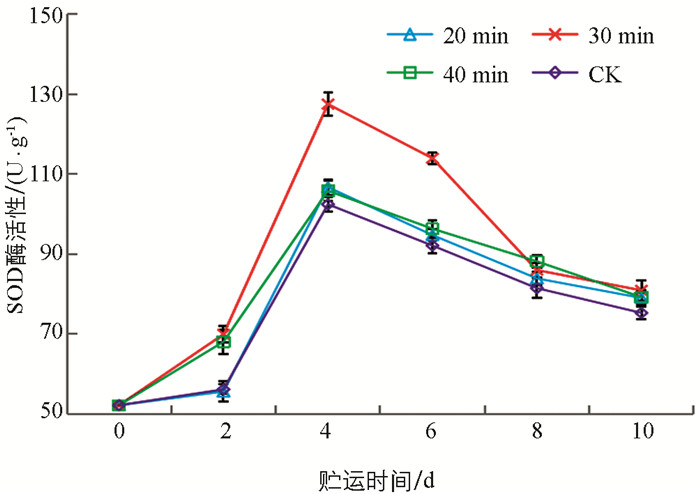
SOD酶在防止细胞受到过量活性氧(ROS)损伤方面发挥着重要作用,提高SOD水平能更好地保护羊肚菌免受氧化胁迫[33]. 图 5显示,UVC处理可有效增加SOD酶活性,30 min UVC处理2 d,4 d,6 d,8 d,10 d分别比CK对照提高24.54%,24.46%,23.72%,5.54%和7.49%,特别是4 d,6 d时30 min UVC处理与20 min和40 min UVC处理差异有统计学意义(p<0.05). UVC处理贮运后期与对照差异无统计学意义.
-
CAT酶作为重要的H2O2清除剂,其活力直接反映了细胞免受活性氧损害的程度[34]. 图 6显示,UVC处理相比CK对照可明显提高其活力,30 min UVC处理2 d,4 d,6 d,8 d,10 d分别比对照提高3.45%,23.69%,5.64%,15.24%和41.16%,其中以4 d,30 min UVC处理效果与对照差异有统计学意义(p<0.05),而20 min和40 min UVC处理与对照差异无统计学意义.
-
H2O2,MDA,超氧阴离子自由基均是反映植物过氧化程度的指标之一[35]. MDA是衡量植物衰老细胞膜透性的一个重要指标[36],为植物衰老生理和抗性生理研究常用的检测指标. 图 7显示,整个贮运过程MDA质量分数一直呈上升趋势,UVC处理可延缓其累积速率,其中,30 min UVC处理2 d,4 d,6 d,8 d,10 d分别比CK对照减少6.31%,11.36%,10.02%,5.61%和13.80%,优于20 min和40 min UVC处理.
2.1. UVC不同处理时间对生鲜羊肚菌感官品质的影响
2.2. UVC不同处理时间对生鲜羊肚菌褐变度的影响
2.3. UVC不同处理时间对生鲜羊肚菌可溶性固形物含量的影响
2.4. UVC不同处理时间对生鲜羊肚菌可溶性蛋白质质量分数的影响
2.5. UVC不同处理时间对生鲜羊肚菌SOD酶活性的影响
2.6. UVC不同处理时间对生鲜羊肚菌CAT酶活性的影响
2.7. UVC不同处理时间对生鲜羊肚菌MDA质量分数的影响
-
羊肚菌品质劣变的表象为菌柄褐度、菌盖萎缩、菌盖褶皱脱落以及内容物外溢,与活性氧自由基动态平衡被破坏[37],活性氧自由基积累,MDA等有害物质产生密切相关[38]. SOD酶是机体清除活性氧的第一道防线,催化超氧化物的歧化反应,增强植物在逆境胁迫下的耐受能力,CAT酶则起协同增效作用. UVC杀菌保鲜符合当前消费者对营养、安全、绿色、天然食物的追求. UVC处理能降低石榴籽粒冷藏过程中的质量损失率、腐烂率及相对电导率,延缓总可滴定酸含量的骤变期,使籽粒中各有机酸及维生素C含量维持在较稳定的水平[39];能延缓龙眼果实失质量、可溶性固形物和可溶性蛋白质量分数的降低,减缓维生素C消耗,抑制MDA质量分数和多酚氧化酶(PPO)活性的积累,维持整个贮藏期间较高的SOD酶活性[40];可有效延长黄蘑菇保鲜期,延缓腐败变质、菌体自溶,抑制与酶促褐变密切相关的PPO酶、苯丙氨酸解氨酶(PAL)活性[41];酸性电解水结合UVC处理能减少“六妹”羊肚菌表面附着的细菌和真菌数,提高SOD酶和维生素C含量,降低PPO酶、过氧化物酶(POD)活性,改善褐变及质地软化程度[16],显著降低韭菜黄变率和腐烂率[42]. 另外,UVC处理还能提高草莓[43]、桃[44]、山楂[45]、鲜切菠萝块[46]、番茄[47]、香瓜[48]等保鲜效果,充分证明UVC处理对生鲜果蔬贮运保鲜品质保持具有重要意义. 本研究结果表明,30 min UVC处理可提高生鲜羊肚菌感官品质评分,减轻褐变度发生,明显提高可溶性蛋白质量分数,使与活性氧清除相关的SOD酶和CAT酶活性保持较高水平,减轻MDA累积程度,减缓生鲜羊肚菌品质衰败.








 DownLoad:
DownLoad: