-
开放科学(资源服务)标识码(OSID):

-
烟草镰刀菌根腐病是由真菌病原体镰刀菌属(Fusarium)成员引起的土传性病害,已成为烟草种植区的主要病害之一[1]。该病害在河南三门峡(发病率为14%~40%)[2]、云南(发病率为5%)[3]、重庆酉阳(发病率为10%~40%)[4]等多地已有报道,给烟草生产造成了重大损失。深入了解微生物群落在根腐病中的作用,对于制定有效的管理策略以减少作物损失至关重要[5]。在烟草镰刀菌根腐病的防治中,细菌和真菌在根腐病的预防和暴发过程中均起着重要作用。例如,已有研究证明,细菌和真菌群落中特定分类群相对丰度的变化与根系的健康和患病状态有关[6-7]。另有学者调查了微生物群落组成对根系健康的影响,通过调控微生物群落组成来降低镰刀菌根腐病的发病率。例如,芽孢杆菌和木霉生物防治剂等菌剂已被证明可以通过抑制致病真菌的生长来促进植物生长并减少根腐病的发生[8-10]。现阶段,学界在了解微生物在根腐病中的作用方面取得了一定的突破[11-13],但其具体作用机制仍有许多未知之处。近年来,基于复杂理论的微生物网络分析技术在微生物相关研究中日益得到青睐,为理解群落中不同生物之间的复杂关系提供了的有效框架[6, 14]。跨域生态网络分析(IDEN)已被用于解析生态系统中不同生物之间的交互网络,如真菌和细菌[15]。该方法通过构建跨域物种互作网络,解析微生物群落的组成结构和功能关联,既能揭示生态位竞争、关键物种调控等基础机制,又能挖掘具有抗病潜力的功能微生物[16]。
因此,本研究通过网络分析的手段阐明烟草根际显著富集的生物指示类群与镰刀菌属之间的相互作用,旨在为丰富烟草土壤微生物资源研究以及探索针对镰刀菌的拮抗微生物资源提供新的策略和理论支持。
HTML
-
采样地位于陕西省商洛烟区连作烟田,按田间发病情况分为发病地与健康地,两块地连作年限一致。健康地海拔1 113.5 m,34°12′40.81″N,110°12′19.23″E;发病地海拔1 122.1 m,34°12′20.30″N,110°11′50.12″E。采样田土壤类型均为黄壤土,样品于2023年6月采集,烟草品种为“秦烟99”,由陕西省烟草公司提供。
-
土壤样品于烟草团棵期采集。
-
发病地与健康地取4个样方作为区内重复,每个样方内随机选取5株烟,使用直径为10 cm的灭菌铲采集5~10 cm的耕层土壤土样,收集烟草根系,采用抖落法收集烟草根际土,将采集的5株烟的土样去除根系、地膜后混合均匀,迅速装入无菌自封袋,暂存于4 ℃冰盒,带回实验室后放置于-80 ℃冰箱保存,后续进行土壤DNA提取。
-
采用Power Soil® DNA试剂盒提取土壤微生物群落总DNA,通过1%琼脂糖凝胶电泳结合溴化乙锭染色技术(C21H12BrN3)进行DNA纯度检测,使用凝胶成像系统(Bio-Rad,USA)进行可视化分析。
-
DNA提取完成后,使用引物515F(5′-GTGCCAGCMGCCGCGG-3′)和907R(5′-CCGT-CAATTCMTTTRAGTTT-3′)对细菌16S rRNA基因的V4—V5区进行PCR扩增。扩增步骤为:95 ℃预变性2 min,95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s,循环25次,最终在72 ℃条件下延伸5 min。各样本重复3次,并用琼脂糖凝胶(2%)电泳检测同一样品的PCR混合产物,之后回收扩增子。根据Axy-Prep DNA凝胶回收试剂盒(Axygen公司)说明书,对上述PCR产物进行纯化、检测和定量。
-
DNA提取完成后,利用引物ITS1F(5'-CTTGGTCATTTAGAGGAAGTAA-3')、ITS2R(5'-GCTGCGTTCTTCATCGATGC-3') 对真菌ITS1区进行扩增。PCR反应体系和条件:95 ℃预变性3 min,接着95 ℃变性30 s,56 ℃退火30 s,72 ℃延伸40 s,循环35次;最终在72 ℃条件下延伸5 min。扩增完成后用2%琼脂糖凝胶对PCR产物进行电泳检测,采用AxyPrep DNA凝胶回收试剂盒(Axygen公司)切胶。Illumina Miseq PE300测序:参考电泳初步定量结果,将PCR产物用Quanti FluorTM-ST蓝色荧光定量系统进行定量检测,之后将每个样本按要求进行混合。最后,将纯化质量合格的PCR产物委托上海美吉医药生物有限公司进行文库构建及上机测序工作。
-
利用Excel 2016进行数据整理,使用Mothur 1.30计算α多样性指数,群落丰富度指数(包括ACE、Chao和Sobs)和群落多样性指数Shannon,采用Kruskal-Wallis秩和检验进行多组比较,基于Bray-Curtis距离进行PCoA分析。采用IBM SPSS 17.0计算多重比较和独立样本t检验;利用image GP进行微生物ASV数据表基础作图;借助Rstudio进行微生物网络数据分析(R>0.6,p<0.05),网络模型构建,Cytoscape绘制子网络模型。
1.1. 试验方法
1.2. 土壤样品采集与处理
1.2.1. 采集时间
1.2.2. 采集方法
1.3. 测定项目及方法
1.3.1. 土壤微生物DNA提取
1.3.1.1. 细菌DNA测序
1.3.1.2. 真菌DNA测序
1.4. 数据处理
-
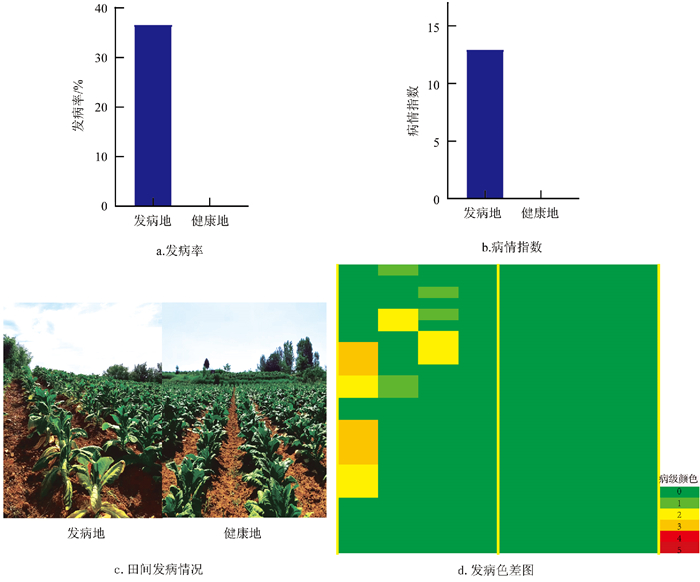
通过对陕西商洛地区健康地与发病地的病害调查数据进行分析(如图 1),结果显示,在旺长期,发病地烟草根腐病发病率为36.67%,病情指数为12.96,而健康地烟株无烟草根腐病发生。这表明健康地与发病地在烟草根腐病发病情况上存在显著差异。
-
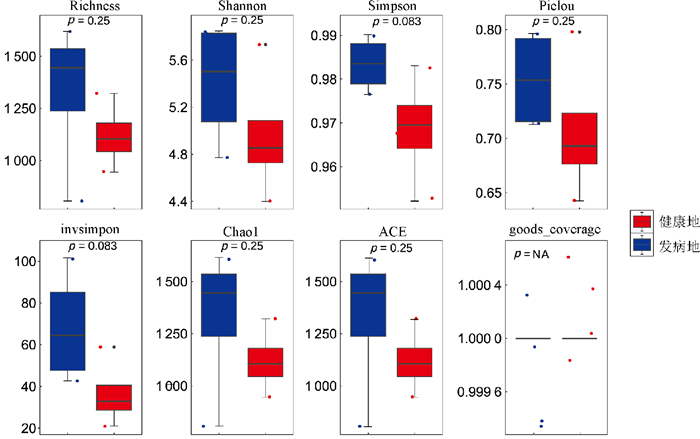
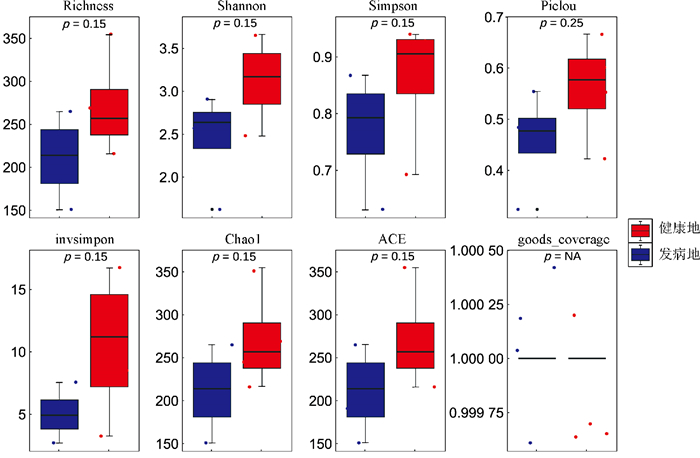
对健康地与发病地烟草根际微生物菌群落α多样性的分析结果表明(图 2和图 3),健康地烟草根际细菌群落的Chao1、Shannon、Richness、ACE指数均低于发病地,但差异水平不显著;而健康地烟草根际真菌群落的Chao1、Shannon、Richness、ACE指数均高于发病地,也未达到显著水平。总体来看,发病地在微生物群落多样性的变化趋势表现为细菌多样性上升,而真菌多样性下降。
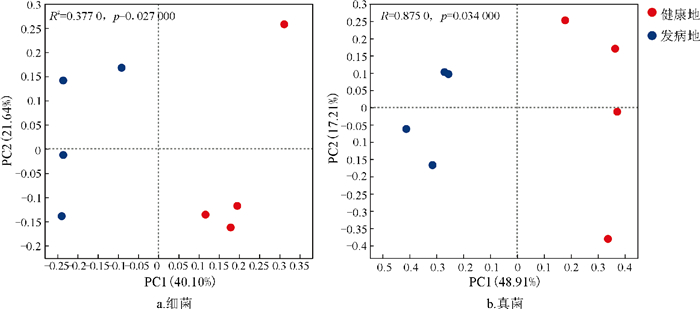
为进一步阐明健康地与发病地烟草根际微生物群落结构的差异情况,本研究采用了基于Bray-Curits距离的主坐标分析(PCoA)方法。在对细菌群落的分析中(图 4a),PC1轴解释了细菌群落总体变化的40.10%,PC2轴解释了细菌群落总体变化的21.64%,且健康地样本点与发病地样本点在PC1轴呈现出明显的分离态势(R2=0.377 0,p=0.027 0),这表明健康地与发病地在烟草根际细菌群落组成上存在显著差异;由图 4b可知,PC1和PC2轴共同解释了真菌群落总体变化的66.12%,健康地样本点与发病地样本点在PC1轴上也呈现分离态势(R2=0.875 0,p=0.034 0),这表明健康地与发病地在烟草根际真菌群落组成上存在显著差异。
-
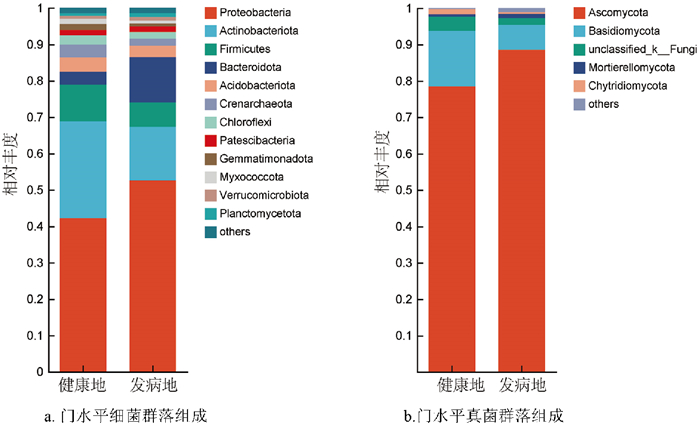
健康地与发病地烟草根际土壤在门水平的细菌群落组成如图 5a所示,健康地根际土壤中的优势菌门为变形菌门(Proteobacteria,42.24%)、放线菌门(Actinobacteriota,26.60%)、厚壁菌门(Firmicutes,10.09%)、酸杆菌门(Acidobacteriota,3.95%)、拟杆菌门(Bacteroidota,3.57%)、泉古菌门(Crenarchaeota,3.45%)、绿弯菌门(Chloroflexi,2.62%)、芽单胞菌门(Gemmatimonadota,1.60)、黏细菌门(Myxococcota,1.50%)、Patescibacteria(1.46%);发病地根际土壤中的优势菌门为变形菌门(Proteobacteria,52.62%)、放线菌门(Actinobacteriota,14.76%)、拟杆菌门(Bacteroidota,12.51%)、厚壁菌门(Firmicutes,6.65%)、酸杆菌门(Acidobacteriota,3.30%)、泉古菌门(Crenarchaeota,1.95%)、绿弯菌门(Chloroflexi,1.89%)、Patescibacteria(1.52%)、浮霉菌门(Planctomycetota,1.08%)、疣微菌门(Verrucomicrobiota,1.04%)。健康地与发病地在门水平的真菌群落组成相似,但相对丰度存在差异(图 5b),健康地根际土壤中的优势菌门为子囊菌门(Ascomycota,78.47%)、担子菌门(Basidiomycota,15.27%)、unclassified_k_ _Fungi(3.90%)、壶菌门(Chytridiomycota,1.33%);发病地根际土壤中的优势菌门为子囊菌门(Ascomycota,88.56%)、担子菌门(Basidiomycota,6.86%)、unclassified_k_ _Fungi(1.82%)、被孢霉门(Mortierellomycota,1.19%)。综上所述,无论是真菌群落还是细菌群落,健康地烟草根际的第一优势菌门相对丰度均低于发病地,其中,健康地放线菌门相对丰度显著高于发病地,而拟杆菌门(Bacteroidota)的相对丰度显著低于发病地,这表明烟草根际的部分微生物群落相对丰度表现出了较大差异。
-
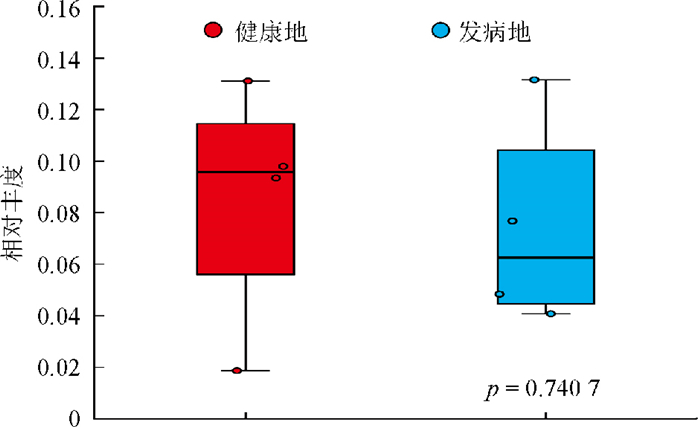
图 6展示了病原菌属在两个地域间的相对丰度情况,结合群落组成分析和田间病害症状,本研究的根腐病菌被鉴定为镰刀菌。值得注意的是,致病真菌镰刀菌属(Fusarium)在健康地和发病地中均存在,其在健康地中的相对丰度为8.508%,略高于发病地的7.418%,但这一差异未达到显著性水平(p=0.740 7)。
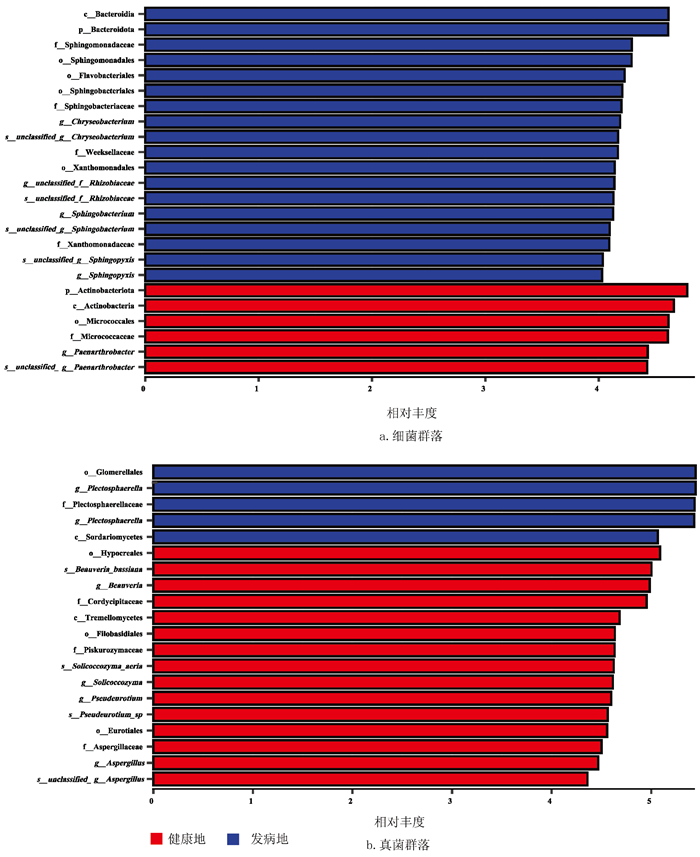
利用LEfSe分析不同处理下最能解释组间差异的物种特征,以及这些特征对组间差异的影响程度,即筛选根际显著富集的生物学标识(biomarker),健康地与发病地根际细菌显著富集的优势类群见表 1。结果表明,在LDA≥4.0的条件下,烟草根际细菌群落发病地共筛选出18个优势类群,健康地共筛选出6个优势类群。在属水平上,金黄杆菌属(Chryseobacterium,LDA=4.19)、unclassified_f_ _Rhizobiaceae(LDA=4.14)、鞘氨醇杆菌属(Sphingobacterium,LDA=4.13)和鞘脂单胞菌属(Sphingopyxis,LDA=4.03)在发病地烟草根际中显著富集;类节杆菌属(Paenarthrobacter,LDA=4.43)在健康地烟草根际中显著富集(图 7a)。同时,在LDA≥4.0的条件下,烟草根际真菌群落发病地共筛选出5个优势类群,健康地共筛选出15个优势类群。在属水平上,褶球壳属(Plectosphaerella,LDA=5.43)在发病地烟草根际中显著富集;白僵菌属(Beauveria,LDA=4.98)、嗜盐酵母属(Solicoccozyma,LDA=4.62)、Pseudeurotium(LDA=4.60)和曲霉属(Aspergillus,LDA=4.47)在健康地烟草根际中显著富集(图 7b)。LEfSe分析结果进一步展示两块地域之间属水平上的生物学标识,如细菌群落中金黄杆菌属Chryseobacterium、unclassified_f_ _Rhizobiaceae、鞘氨醇杆菌属(Sphingobacterium)、鞘脂单胞菌属(Sphingopyxis)和真菌群落褶球壳属(Plectosphaerella)是发病地烟草根际属水平的生物学标识,而细菌属褶球壳属(Paenarthrobacter)和真菌属白僵菌属(Beauveria)、嗜盐酵母属(Solicoccozyma)、Pseudeurotium、曲霉属(Aspergillus)是健康地烟草根际属水平下的生物学标识。
-
属水平下微生物群落的网络分析结果表明,发病地的细菌和真菌网络的规模(即节点和边的数量)减小,表明健康地土壤中微生物群落的域内网络模型具有更高的稳定性(图 8a,8b,8c,8d),对外界侵染具有更高的抵抗力。真菌子网络模型结果表明,在发病地中,Rhodotorula、Dendryphion、unclassified_f_ _Ceratobasidiaceae与镰刀菌属存在显著的负相关关系,Podospora、unclassified_f_ _Hyponectriaceae与镰刀菌属存在显著的正相关关系(p<0.05)(图 8e);而在健康地中,Ganoderma、Knufia、Mortierella、Penicillium、Pyrenochaeta、Tausonia、unclassified_k_ _Fungi、Solicoccozyma、Chalara、Chrysosporium与镰刀菌属存在显著的负相关关系(p<0.05),Pseudeurotium与镰刀菌属存在显著的正相关关系(p<0.05),有趣的是,上述与镰刀菌属呈负相关的菌属与Pseudeurotium菌属也表现出显著的负相关关系(图 8f)。
细菌-真菌群落的跨域网络模型结果表明,健康样品网络的节点和链接数(200个真菌节点和200个细菌节点,248 3个链接),发病地网络(191个真菌节点和200个细菌节点,259 9个链接),镰刀菌感染后,细菌-真菌跨域网络节点的数量减小,但是边的数量增加(图 9a和图 9b)。在跨域网络模型中,健康地细菌-真菌跨域网络中边的负相关性更低(6.4%),而发病土壤细菌-真菌群落跨域网络边的负相关性为8.5%。
细菌-真菌跨域子网络模型结果表明,在发病地中,细菌群落存在10个与镰刀菌属存在显著相关性的细菌属,其中只有unclassified_c_Acidimicrobiia和镰刀菌属呈现出显著负相关,其余均为正相关,分别为Taibaiella、norank_f_ _Rhodocyclaceae、类诺卡氏菌属(Nocardioides)、norank_f_ _Fimbriimonadaceae、鞘脂单胞菌属(Sphingopyxis)、Shimazuella、分枝杆菌属(Mycobacterium)、苍白杆菌属(Ochrobactrum)、unclassified_f_ _Pseudonocardiaceae,这表明在发病地中,与镰刀菌属相关的细菌群落与镰刀菌属存在更多的合作关系(图 9c)。在健康地中,细菌群落存在9个与镰刀菌属存在显著相关性的细菌属,其中8个细菌属均与镰刀菌属存在显著的负相关关系,分别为无色杆菌属(Achromobacter)、unclassified_f_ _Microbacteriaceae、unclassified_f_ _Blastocatellaceae、Peredibacter、噬冷杆菌属(Pedobacter)、norank_f_ _Saprospiraceae、金黄杆菌属(Chryseobacterium)、寡养单胞菌属(Stenotrophomonas),这表明健康地中与镰刀菌属相关的细菌群落与镰刀菌属存在更多的拮抗关系(图 9d)。
2.1. 健康地与发病地的发病差异
2.2. 健康地与发病地微生物群落多样性分析
2.3. 发病地与健康地门水平下微生物群落组成
2.4. 发病地与健康地属水平下微生物群落富集情况
2.5. 发病地与健康地属水平上共现网络的差异性
-
土壤对土传病害的抑制作用是作物生态学领域的研究热点之一。抑制性土壤能够抑制病害,并且具有一定的维持能力。Durán的研究结果表明,在抑制性土壤中,病害的减轻并不总是意味着病原体数量减少[17],这也与本研究的结果一致。通过比较发现,健康地与发病地的致病菌属并未表现出显著差异性,但两地的真菌和细菌群落在组成上存在较大差异。在门水平上,健康地的放线菌门相对丰度显著高于发病地,而Bacteroidota的相对丰度显著低于发病地,LEfSe分析结果显示,在属水平上,发病地烟草根际的生物学标识包括细菌属中的Chryseobacterium、unclassified_f_ _Rhizobiaceae、Sphingobacterium、Sphingopyxis和真菌属Plectosphaerella;而细菌属Paenarthrobacter和真菌属Beauveria、Solicoccozyma、Pseudeurotium、Aspergillus是健康地烟草根际的生物学标识。这与前人的研究结果相近,表明致病菌的存在可以限制微生物多样性并重塑群落结构[18, 19]。值得注意的是,Paenarthrobacter因具有较强的生物降解活性被认为是开发价值较高的生物功能菌[20-22],Carvajal等[23]研究发现,Solicoccozyma具有促进植物生长的效果,这表明在健康土壤中烟草根际富集着更多对植物有益的功能微生物。
土壤作为病原菌侵染植株的微生态环境,其微生物群落通过竞争、协作和不相关3种互作模式动态调控病原菌定殖过程[24]。其中,微生物间的竞争压力被认为是影响镰刀菌侵染植物的重要因素[25]。已有研究通过对比发病地与健康地的微生物群落特征,初步揭示了微生物群落与镰刀菌根腐病发生的关联性[6, 26],但涉及细菌和真菌的复杂相互作用的研究却鲜有报道。本研究结果表明,在跨域网络模型中,健康地细菌-真菌跨域网络边的正相关更高,表现出更高的协同趋势。同时,子网络模型结果表明,在发病地的细菌群落中,有10个与镰刀菌属存在显著相关性的细菌属,只有unclassified_c_ _Acidimicrobiia和镰刀菌属呈现出显著负相关,其余均为正相关,这表明在发病地中,与镰刀菌属相关的细菌群落表现出更多的合作关系。在健康地的细菌群落中有9个与镰刀菌属存在显著相关性的细菌属,其中8个细菌属与镰刀菌属存在显著的负相关关系,这表明健康地中与镰刀菌属相关的细菌群落表现出更多的拮抗关系。上述结果表明,在健康地中,镰刀菌面临更大的竞争压力,这可能是两地发病差异的原因。
综上所述,本研究揭示了健康地土壤中存在更丰富的有益微生物群落,并明确了镰刀菌在健康烟地中面临更大的竞争压力,同时指出了造成竞争压力的可能的微生物属,如Solicoccozyma。这些发现为镰刀菌根腐病防治中有益微生物的分离提供了重要参考。此外,研究还强调了需要进一步关注发病地中与镰刀菌属“协作”的微生物群落(如Sphingopyxis)。这些结果对于烟草镰刀菌根腐病的防治具有重要的理论和实践意义。







 DownLoad:
DownLoad: