-
开放科学(资源服务)标志码(OSID):

-
低温噬菌体广泛分布于极地、冰川、高原湖泊、深海及其他各类低温生境中,是地球生物圈最丰富多样的生命形式,同时也是调节各类环境中原核生物群落结构和生态功能最主要的驱动因子之一[1-2]. 近年来,超级耐药细菌的频繁传播和化学农药的滥用,对全世界公共卫生及绿色可持续发展事业造成了日益严重的威胁,迫切需要寻找替代或补充抗生素、化学农药的新技术[3-5]. 噬菌体又称细菌病毒,因其对宿主菌的高度专一性、生物安全性高及在各类环境中广泛存在和持久的增殖能力,在治疗人类和动植物细菌感染方面潜力巨大[6-7],被认为是21世纪重要的战略生物资源[8].
黄杆菌属(Flavobacterium)细菌是非发酵革兰氏阴性杆菌,广泛存在于土壤和水环境中,多为条件致病菌,常引起人和鱼类感染,不仅是造成医院感染的主要病原菌之一,还给水产养殖业造成了重大的经济损失[9]. 当前主要是使用化学药物对抗由细菌引起的水产病害,这不仅直接导致水产品药物残留的安全问题,而且间接造成水体生态系统失衡和耐药菌株频现等不良问题. 噬菌体的治疗或预防方案为水产养殖及生态系统安全提供了新思路[10-14],如基于噬菌体能够降解致病菌的噬菌体鸡尾酒疗法,或噬菌体所编码裂解蛋白(穿孔素或裂解酶)的开发应用已得到广泛关注等[6, 15].
高原湖泊作为一类古老的生态系统,低温菌及其噬菌体是其中主要的生物类群,独特的基因组成、进化特征及在低温生境中不可替代的生态功能使其备受关注. 挖掘高原湖泊中的低温菌及噬菌体资源,不仅对了解细菌-噬菌体的协同进化关系有重要意义,而且对发掘新物种和基因资源有重大实践价值. 本文从喀拉库勒湖分离获得两株能侵染同一宿主黄杆菌的低温噬菌体,对其电镜形态、生物学特性、基因组酶切图谱以及理化因素敏感性等进行了分析,以期为后续噬菌体与宿主菌协同进化、噬菌体编码裂解蛋白开发利用奠定理论基础.
HTML
-
2018年10月,于东帕米尔高原喀拉库勒湖(38°25′N,75°02′E;海拔3 618 m)沿湖采集水样,封装于50 mL离心管中于低温下带回实验室. 采样点水温2~4 ℃,pH值为7.8~8.4.
-
宿主菌及噬菌体分离采用PYGV培养基[16],半固体培养基使用熔点更低的琼脂糖作凝固剂(终浓度为0.4%). 细菌基因组提取试剂盒、Taq酶、限制性内切酶等均购自宝日医生物技术(北京)有限公司.
-
将不同采样点湖水样混匀后10倍序列稀释,取200 μL不同稀释度水样涂布于PYGV平板上,15 ℃培养3~7 d,依据平板上菌落形态特征进行划线纯化,获得纯菌株.
-
采用双层琼脂平板法分离噬菌体[16],依据噬菌斑形态特征挑取单斑纯化,待噬菌斑形态一致时刮取上层琼脂接入PYGV液体培养基中进行增殖培养,培养液经13 000 r/min离心10 min,上清液即噬菌体液.
-
将纯化的噬菌体液与全部分离菌株分别进行双层平板实验,检验是否有噬菌斑产生,以确定已分离噬菌体是否存在其他宿主菌.
-
采用细菌基因组提取试剂盒提取宿主菌DNA,以通用引物27 F(5′-AGAGTTTGATCCTGGCTCAG-3′)和1 541 R(5′-AAGGAGGTGATCCAGCCGCA-3′)[17]扩增16S rRNA基因序列. PCR反应条件参照文献[16]进行,PCR产物经1%琼脂糖凝胶电泳检测后送生工生物工程(上海)股份有限公司测序,所得16S rRNA基因序列与GenBank数据库中已知序列进行BLAST比对分析,用ClustalX软件和MEGA 6.0构建系统发育树,确定分类地位.
-
取效价约1×108 PFU/mL的噬菌体液100 mL,采用超滤离心管(millipore,10 kD)浓缩至1 mL. 吸取20 μL滴于铜网上,10 min后用吸水纸吸干多余液体,于铜网上加1滴2%,pH值为4.5的磷钨酸染色5~10 min,待铜网自然干燥后用透射电子显微镜(JEM1200EX)观察噬菌体的粒子形态.
-
将宿主菌接种于PYGV平板上,分别于4,10,15,20,25,30,37和42 ℃培养3 d,定时观察菌落生长情况. 依据菌落生长特征设定培养温度范围,在装液量和接种量都统一的条件下采用液体振荡培养,以浊度法绘制不同温度下宿主菌的生长曲线,确定其生长温度范围,3次重复.
-
取100 μL噬菌体液与300 μL对数生长期的宿主菌悬液混合进行双层平板实验,分别置于宿主菌各生长温度下培养3 d,观察噬菌斑及菌膜生长情况,确定噬菌体侵染宿主菌的温度范围,3次重复.
-
将噬菌体液效价调整为1×105 PFU/mL,分别取2 mL于40,50,60和70 ℃水浴处理,自0时起每10 min取样测定不同温度下的噬菌体效价. 各处理均3次重复,以未处理的噬菌体液作对照,分析不同温度处理下噬菌体的稳定性.
-
同1.6.1,分别取500 μL噬菌体液加入到4.5 mL不同pH的缓冲液中,室温处理1 h,12 000 r/min离心5 min,测定上清液中噬菌体效价. 各处理均3次重复,以未处理的噬菌体液作对照.
-
氯仿敏感性:同1.6.1,分别取2 mL噬箘体液,加入0.5 mL氯仿(终浓度为20%),混匀后于室温下处理10 min,经12 000 r/min离心5 min,取上清液测定噬菌体效价,以未处理的噬菌体液作对照.
蛋白酶K敏感性:同1.6.1,取2 mL噬菌体液至5 mL EP管中,加入蛋白酶K至终浓度为1 mg/mL,在56 ℃水浴处理30 min,经12 000 r/min离心5 min后测定上清液中噬菌体效价,以未处理的噬菌体液作对照.
表面活性剂敏感性:同1.6.1,取2 mL噬菌体液,加入表面活性剂SDS至终浓度为0.1%,于56 ℃处理10 min后离心,测定上清液中噬菌体效价. 各取2 mL噬菌体液,加入0.3%的表面活性剂Triton X-100于室温下处理10 min,离心测定上清液中噬菌体效价. 各处理均作3次重复,以未处理的噬菌体液作对照.
-
以噬菌体效价(PFU/mL)与宿主菌悬液(CFU/mL)分别为0.001,0.01,0.1,1,10和100的比例混匀,终体积为30 mL,15 ℃,180 r/min振荡培养4 h,12 000 r/min离心5 min,测定上清液中噬菌体效价. 各处理均3次重复,计数噬菌斑形成数量最多的“比例”即为OMOI.
-
将噬菌体KH-sph01和KH-sph02分别以最佳感染复数与对数期宿主菌悬液混匀,于15 ℃下静置,分别在0,2,4,6,8,10,15,20和30 min时取样100 μL,12 000 r/min离心5 min,测定上清液中未被吸附的噬菌体粒子数量(PFU/mL). 以取样时间为横坐标,测得的噬菌体效价为纵坐标,绘制曲线.
-
取对数期宿主菌悬液10 mL,调整吸光度OD600为0.6(活细胞数约1×108 CFU/mL). 按最佳感染复数加入噬菌体液混合,15 ℃吸附10 min,以12 000 r/min离心5 min弃上清以去除未被吸附的噬菌体粒子,加入10 mL的PYGV液体重悬沉淀后于15 ℃,180 r/min振荡培养. 自0时起每10 min取样1次,12 000 r/min离心测定上清液中噬菌体效价. 以未加宿主菌的噬菌体液和未加噬菌体的宿主菌悬液作对照,以取样时间为横坐标,噬菌体效价的对数值为纵坐标绘制一步生长曲线.
-
将噬菌体KH-sph01和KH-sph02与宿主菌悬液按最佳感染复数混匀接入PYGV培养基,15 ℃,180 r/min振荡培养2 d. 取培养液1 L于12 000 r/min离心10 min,向上清液中加入脱氧核糖核酸酶Ⅰ(DNaseⅠ)和核糖核酸酶A(RNase A)至终浓度为1 μg/mL,37 ℃水浴30 min后加入NaCl至终浓度为1 mol/L,冰浴1 h后于4 ℃,12 000 r/min离心10 min. 向上清液中加入PEG 8000至终浓度为10%,充分溶解后于4 ℃静置过夜,经4 ℃,12 000 r/min离心10 min弃上清,用4 mL的SM缓冲液重悬沉淀,加入等体积氯仿涡旋振荡2 min,8 000 r/min离心10 min收集水相,即得浓缩的噬菌体液.
-
取浓缩的噬菌体液500 μL,加入乙二胺四乙酸(EDTA)至终浓度为20 mmol/mL、蛋白酶K(终浓度为50 μg/mL)和10%SDS(终浓度为50 μL/mL),56 ℃水浴1 h,以酚-氯仿法抽提噬菌体基因组. 12 000 r/min离心5 min收集水相,加入等体积冰冷的异丙醇混匀,12 000 r/min离心10 min,沉淀用1 mL无水乙醇洗涤,12 000 r/min离心10 min,加入50 μL的TE缓冲液溶解沉淀,即得噬菌体DNA. 采用微量紫外分光光度计(Nanodrop 2000)检验纯度和浓度后于-20 ℃保存.
-
分别用DNaseⅠ和RNase A对噬菌体基因组进行酶切,反应体系为10 μL,即基因组1μL,DNaseⅠ或RNase A酶1 μL,10×buffer 1 μL,ddH2O补至10 μL. 37 ℃水浴30 min后采用0.7%琼脂糖凝胶电泳检测,以判断噬菌体基因组的核酸类型. 基因组为DNA的,则继续用EcoRⅠ,BamHⅠ,PstⅠ和XhoⅠ等限制性核酸内切酶酶切分析,以0.7%琼脂糖凝胶电泳检测.
1.1. 样品采集
1.2. 培养基与主要试剂
1.3. 宿主细菌及噬菌体分离
1.3.1. 宿主细菌分离
1.3.2. 噬菌体分离
1.3.3. 噬菌体宿主谱分析
1.4. 宿主菌鉴定与噬菌体电镜形态
1.4.1. 宿主菌16S rDNA基因测序及系统发育分析
1.4.2. 噬菌体形态观察
1.5. 宿主菌生长及噬菌体侵染温度范围
1.5.1. 宿主菌生长温度范围测定
1.5.2. 噬菌体侵染温度范围
1.6. 理化因素对噬菌体的影响
1.6.1. 热稳定性分析
1.6.2. 酸碱耐受性分析
1.6.3. 氯仿、蛋白酶K和表面活性剂对噬菌体的影响
1.7. 噬菌体主要生物学特征分析
1.7.1. 最佳感染复数(Optimal Multiplicity of infection,OMOI)测定
1.7.2. 吸附速率测定
1.7.3. 一步生长曲线测定
1.8. 噬菌体颗粒浓缩、基因组提取与酶切分析
1.8.1. 噬菌体颗粒浓缩
1.8.2. 噬菌体基因组提取
1.8.3. 噬菌体基因组酶切
-
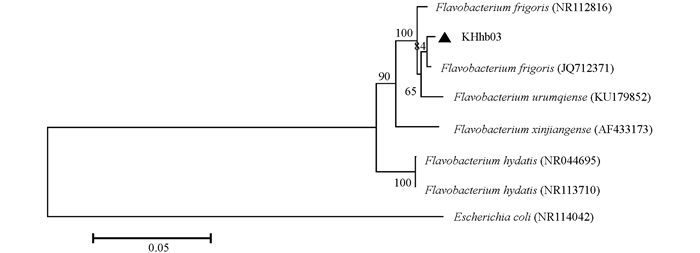
从喀拉库勒湖水样中共分离得到低温细菌43株,以此为宿主,分离得到两株能侵染同一宿主菌的低温噬菌体. 结合16S rRNA序列比对及系统发育分析(图 1),发现该宿主菌与Flavobacterium frigoris (JQ712371)相似度最高,鉴定其为黄杆菌属细菌,命名为Flavobacterium sp.KHhb03,GenBank序列登录号MT919896.
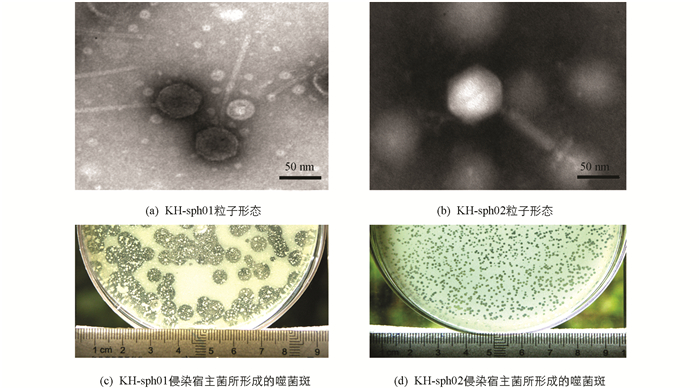
两株噬菌体侵染宿主菌后于双层平板上形成大(直径约3~5 mm、边缘不光滑不整齐)、小(直径约1~2 mm、边缘整齐光滑)两种形态的噬菌斑(图 2). 透射电镜观察发现两株噬菌体粒子形态明显不同,噬菌斑较大的一株头部呈正多面体对称,直径约55~60 nm,尾管细长,约230~250 nm,从形态上初步鉴定为长尾噬菌体科(Siphoviridae),命名为KH-sph01. 噬菌斑略小的一株噬菌体同为头尾复合结构,正多面体头部直径略大,约70~75 nm,尾管粗壮呈针管状,长约120~150 nm,尾管直径约20~25 nm,将其鉴定为肌尾噬菌体科(Myoviridae),命名为KH-sph02. 经宿主谱分析,两株噬菌体仅对宿主菌Flavobacterium sp.KHhb03敏感,表现为烈性噬菌体.
-
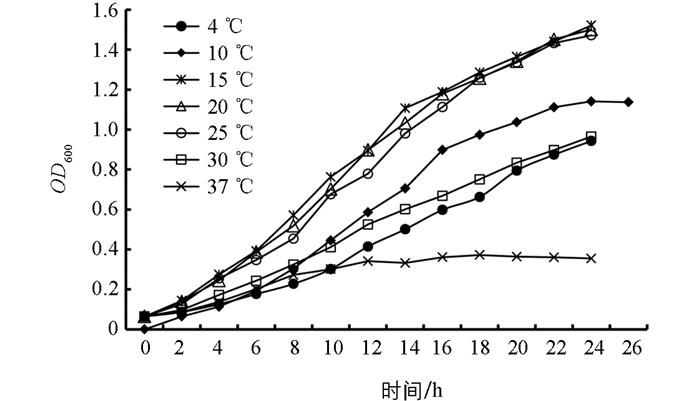
宿主菌Flavobacterium sp.KHhb03经平板培养,发现可在4~37 ℃生长,40 ℃时菌落生长不明显. 不同温度下的生长曲线显示(图 3),该菌株在37 ℃时生长不明显,培养8 h后培养液OD600几乎不再变化;4 ℃和30 ℃时,该菌株生长较缓慢,代时明显延长. 宿主菌Flavobacterium sp.KHhb03最适生长温度为15~20 ℃,属于耐冷菌.
由表 1可见,长尾噬菌体KH-sph01在4~25 ℃培养时能于双层平板上产生清晰的噬菌斑,而肌尾噬菌体KH-sph02侵染温度范围为4~30 ℃,但在30 ℃时的噬菌斑数量低于4~25 ℃.
-
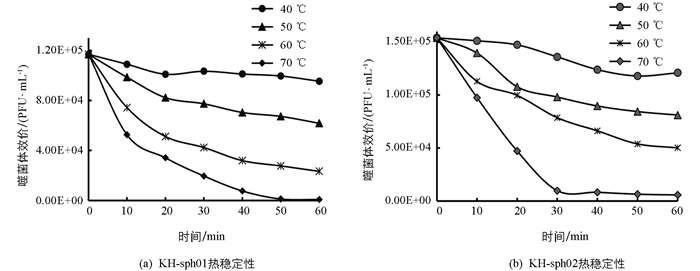
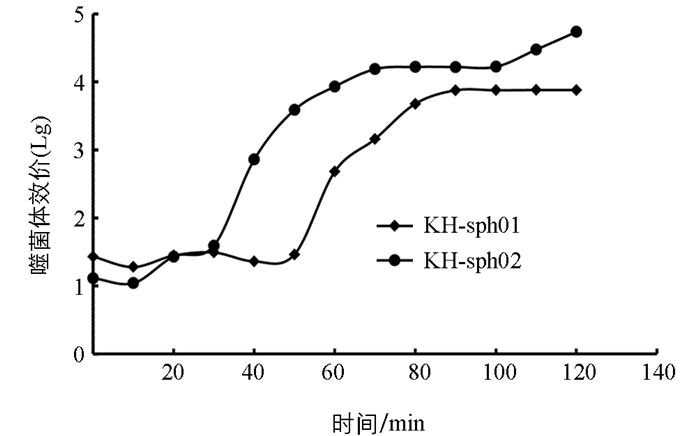
两株噬菌体均表现出一定的热不稳定性(图 4). 40 ℃处理60 min,KH-sph01和KH-sph02效价仍分别有原效价的82%和79%. 50 ℃处理60 min,两株噬菌体均有超过50%的粒子失活. 但随温度继续升高,两株噬菌体耐热性显著降低. 70 ℃处理10 min,两株噬菌体效价仅为原效价的45%和63%;70 ℃处理60 min,两株噬菌体均基本失活. 由此表明,两株噬菌体均对热敏感,但具备一定的耐热性.
-
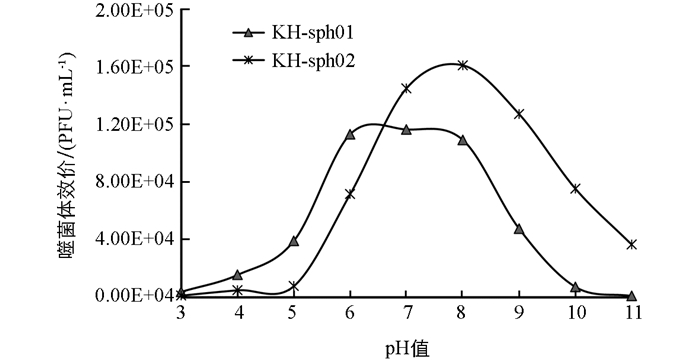
KH-sph01和KH-sph02在pH值为7~8范围内均较稳定(图 5),再侵染能力不受影响. 相比而言,KH-sph01比KH-sph02更耐酸,且对pH的耐受范围更宽,其在pH值为6时侵染能力不受影响,在pH值为5时也有34%的噬菌体存活. 而KH-sph02在pH值为7~9范围内最稳定,在pH值为6和pH值为10时也分别能保持47%和49%的存活率,KH-sph02更能耐受碱性环境.
-
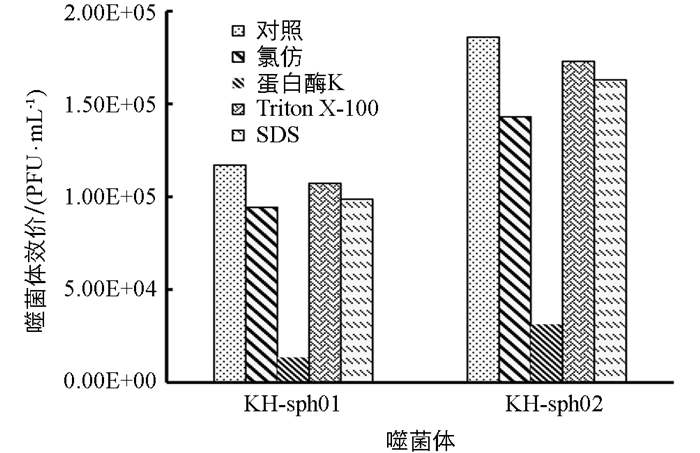
经氯仿处理后(图 6),KH-sph01和KH-sph02的效价相比对照组仍有80.6%和85.3%,表明两株噬菌体均对氯仿不敏感,说明两株噬菌体衣壳组分中均无脂质包膜成分.
经蛋白酶K处理后,KH-sph01和KH-sph02效价仅为对照组的10%和17%,说明两株噬菌体均对蛋白酶K敏感.
KH-sph01和KH-sph02对表面活性剂(SDS和Triton X-100)均表现不敏感,且敏感程度差异无统计学意义,经处理后均能保持85%的侵染活性.
-
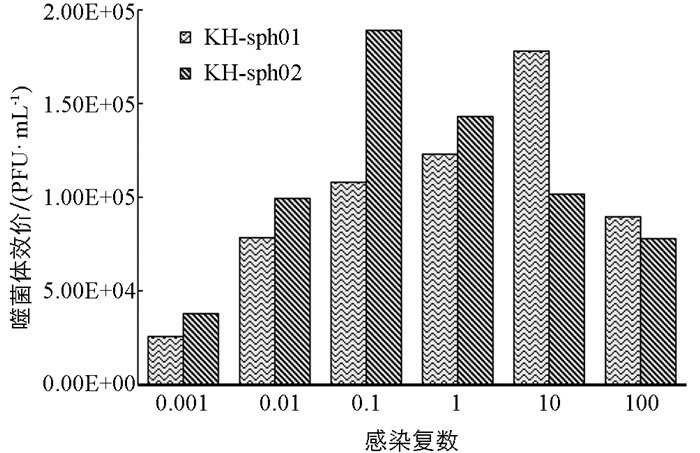
由图 7可见,KH-sph01与宿主细胞比例为10时,培养液中所获子代噬菌体数量最多,而KH-sph02与宿主细胞比例为0.1时,测得培养液中噬菌体效价最高,说明KH-sph01与KH-sph02感染宿主菌Flavobacterium sp.KHhb03的最佳感染复数分别为10和0.1.
-
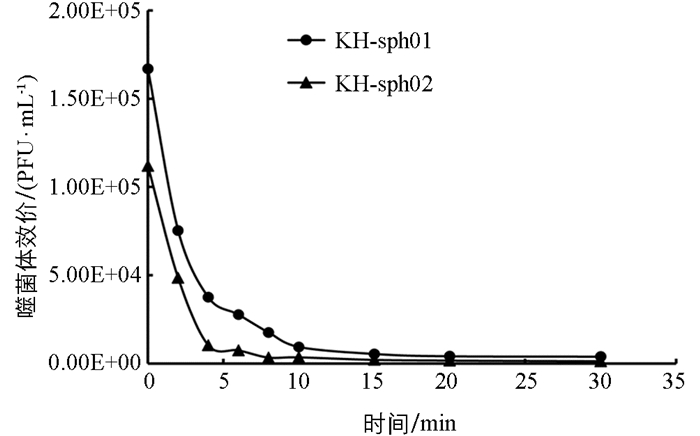
吸附是噬菌体感染宿主细胞的第一步,也是其能否成功感染并实现增殖的必要前提. 由图 8可见,两株噬菌体几乎都能在10 min内完成对宿主菌的吸附. 吸附2 min时,KH-sph01就有超过50%的噬菌体粒子完成吸附,而KH-sph02的吸附速率更强,2 min后有超过55%的噬菌体粒子完成吸附,4 min时有90%的噬菌体粒子吸附到了宿主细胞上,说明两株噬菌体吸附速率均较强.
-
一步生长曲线能反映噬菌体感染宿主细胞到增殖裂解的时间,并由此计算噬菌体的裂解量(B). KH-sph01感染宿主菌的潜伏期约50 min,裂解期约30 min;KH-sph02感染宿主菌的潜伏期和裂解期分别约30 min和40 min(图 9). 依据公式
式中,P表示裂解末期噬菌体效价(PFU/mL),C表示感染初期宿主菌浓度(CFU/min),可得出KH-sph01和KH-sph02的裂解量分别为3.16个和10.22个.
-
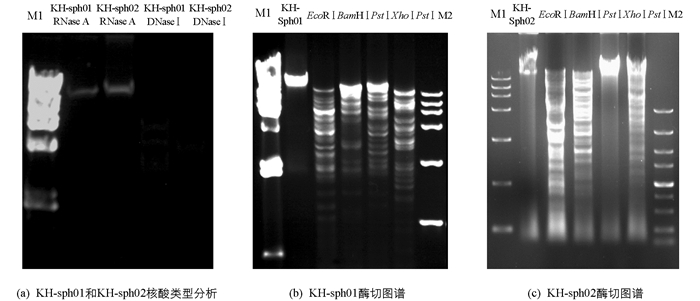
将KH-sph01和KH-sph02的基因组分别用DNaseⅠ和RNase A酶切(图 10a),两株噬菌体均能被DNaseⅠ降解,而RNase A酶解无影响,说明两株噬菌体核酸类型均为dsDNA. 两株噬菌体均能被4种限制性内切酶切割成大小不同的片段(图 10b和10c),进一步说明KH-sph01和KH-sph02基因组均为dsDNA. 综合分析不同酶切图谱,初步估算出KH-sph01的基因组DNA大小为60~65 kb,KH-sph02的基因组DNA大小为40~45 kb.
2.1. 宿主菌分离及其噬菌体初步鉴定
2.2. 宿主菌生长及噬菌体侵染温度范围
2.3. 理化因素对噬菌体的影响
2.3.1. 热稳定性分析
2.3.2. 酸碱耐受性分析
2.3.3. 氯仿、蛋白酶K和表面活性剂对噬菌体的影响
2.4. 两株噬菌体的主要生物学特征
2.4.1. 最佳感染复数
2.4.2. 吸附速率
2.4.3. 一步生长曲线
2.5. 噬菌体基因组提取与酶切分析
-
噬菌体特别是低温噬菌体在防治水产病原菌、动植物病害、维持环境生态平衡等方面的应用层出不穷[18-21],而从环境中分离筛选高效裂解性噬菌体是其开发应用的前提条件. 本研究从喀拉库勒湖分离到的两株低温噬菌体KH-sph01和KH-sph02,虽然能侵染同一宿主菌Flavobacterium sp.KHhb03,但形态各异,在双层平板上形成大、小两种不同形态的噬菌斑,分属于长尾噬菌体科和肌尾噬菌体科. 而李建凯[22]分离的侵染同一宿主荧光假单胞菌Pseudomonas fluorescens W-6的两株低温噬菌体VW-6S和VW-6B均属于典型的长尾噬菌体科,其头部直径和尾部长度分别为66.7/233.3 nm和61.1/166.7 nm,这与本文的发现明显不同. 噬菌体对宿主菌存在高度专一性,一般认为仅有少数形态较为相似的噬菌体才能侵染相同宿主,而本研究KH-sph01和KH-sph02在形态学上却分属于两个不同的噬菌体科,说明自然界中能侵染相同宿主的噬菌体普遍存在,且可能与形态特征无直接关系.
黄杆菌属细菌作为条件致病菌,常引起水产养殖业中细菌性鱼类疾病(如嗜冷黄杆菌Flavobacterium psychrophilum,柱状黄杆菌Flavobacterium columnare等),对鱼类特别是冷水鱼类的人工养殖影响巨大. 噬菌体疗法是近年来由于抗生素滥用及化学农药、重金属等污染频现而产生的一种新型生物防治技术. 它是通过定向筛选、纯化特定致病细菌的专属烈性噬菌体,经人工富集培养或改造后添加到致病细菌污染的生态环境介质中,从而定向灭活致病细菌的治疗方式[23-24]. 分离和筛选特定目标致病细菌的专属噬菌体是噬菌体疗法在不同环境中开展生物防治应用的必要前提. Stenholm等[25]从丹麦一个虹鳟鱼养殖场水样中分离得到22株黄杆菌噬菌体,它们在形态学上和基因组大小上均不尽相同,其中5株(FpV-5,FpV-6,FpV-8,FpV-9和FpV-11)具有较广的宿主谱,可感染多株本地分离的嗜冷黄杆菌,具有良好的开发应用价值. 本研究的宿主菌KHhb03与Flavobacterium frigoris(JQ712371)相似度最高,且在前期的研究中发现黄杆菌属是该区域环境中的优势菌群[26],这对作为噬菌体疗法在区域环境中靶向灭活致病黄杆菌属细菌提供了良好的材料.
系统分析新发现噬菌体的生物学特性是认识该噬菌体的基本前提,更是噬菌体资源开发应用的必要基础. 感染复数是研究噬菌体感染宿主细菌与子代个体产出之间的量效关系的重要生物学指标. KH-sph01和KH-sph02侵染宿主菌的最佳感染复数分别为10和0.1,这与噬菌体VW-6S和VW-6B侵染宿主荧光假单胞菌W-6(0.1和0.01)明显不同,说明即便是不同噬菌体侵染相同宿主菌,其最佳感染复数也可能不尽相同. 一步生长曲线可间接反映噬菌体与宿主菌的相互作用及开发应用潜力,KH-sph01和KH-sph02侵染宿主菌KHhb03的潜伏期和裂解期各不相同,裂解量分别为3.16个和10.22个,间接反映了两株噬菌体与宿主菌相互作用的复杂性. 而在实际操作中也发现,KH-sph01在纯培养条件下难以获得较高的效价,推测在纯培养条件下,随着噬菌体效价增加,宿主菌KHhb03极易对KH-sph01产生高水平抗性以避免其重新感染,这可能是因为纯培养条件下,噬菌体KH-sph01由烈性转变成了温和性,需要进一步验证.
与众多已报道的低温噬菌体一样,KH-sph01和KH-sph02可以在4 ℃时侵染宿主并产生噬菌斑,但分别在超过25 ℃和30 ℃时失去侵染活性,这也印证了两株噬菌体的低温特性. 两株噬菌体均对热敏感,随着温度升高,噬菌体迅速失活. 而Wells等[27]在分析低温噬菌体9A时发现,当温度超过35 ℃时即可使其瞬时灭活,表明低温噬菌体对温度敏感,可因高温而失去活性. 究其原因可能与噬菌体结构蛋白有关,同样也与其生存环境关系巨大,这与以前关于低温噬菌体的报道相符[22, 27-29]. 推测低温噬菌体普遍存在热不稳定性,但在进化过程中因环境不同而产生了不同的适应策略.






 DownLoad:
DownLoad: