-
开放科学(资源服务)标识码(OSID):

-
抗生素在农业、水产养殖以及保障人们健康方面发挥着关键作用[1]. 当前,渔业和畜牧业对抗生素的需求量大幅增加[2]. 例如,美国每年约使用1.6万t抗菌化合物,其中约70%用于非药物治疗. 澳大利亚、新西兰、加拿大和欧盟国家也观察到类似的抗生素使用模式[3]. 水体中的抗生素通常来源于城市污水、制药业废水、畜牧业粪便废水、垃圾处理过程中的渗滤液和水产养殖过程中的饵料添加剂等[4]. 因此,河流、湖泊、地下水和废水处理厂废水中抗生素的检出率较高,其质量浓度变化从1 ng/L到1 000 μg/L[1, 5]. 残留的抗生素不仅会毒害水生生物,还会造成耐药性问题[5-6]. 当前,抗生素污染已引起全球科学界的关注.
光合自养型微藻是水生生态系统中的主要初级生产者[7]. 水体中藻类物种丰富,密度及生物量庞大,且藻类对污染物敏感性高,常作为重金属、农药和抗生素等污染物生态风险评估的指示性物种[5, 8]. 抗生素是微生物产生的一类次级代谢产物,可以影响藻类的生长和存活[9]. 其中,链霉素是一种氨基糖苷类抗生素,可以用来控制水果、蔬菜、烟草、观赏植物、池塘和水族箱上的细菌、真菌和藻类[10]. 链霉素能与原核生物的30S核糖体结合,抑制蛋白质的生物合成[11],还能阻碍生物的叶绿体发育,降低原核生物密度并抑制其生长繁殖[12]. Harrass等[13]研究发现,水体中的蓝藻类对链霉素的敏感性较高,其质量浓度变化范围为0.09~0.86 mg/L,绿藻类对链霉素敏感性相对较低,质量浓度变化范围为0.66~37 mg/L. 当前,有关抗生素对水体中浮游植物生态毒性的研究报道较广泛[14-17],而关于抗生素与淡水微藻生理活性的关系、叶绿素荧光的响应机制报道较少. 有研究发现,叶绿素荧光可以反映光合系统Ⅱ(PS Ⅱ)与环境胁迫变化的响应[15-16]. 根据瞬时叶绿素荧光动力学参数变化特征,研究人员发现藻类生理状态与光合作用过程中的电子和光子流动有明显的相关性[15-17]. 因此,本文通过研究链霉素对蓝藻-拟柱孢藻(Raphidiopsis raciborskii)和广布种绿藻-四尾栅藻(Scenedesmus quadricauda)的生长、叶绿素荧光和半致死浓度(EC50)的影响,探究不同门类微藻对链霉素的敏感性和耐受性差异,特别是光合系统Ⅱ对链霉素的响应特征,旨在为藻类毒理学研究提供新的视角,并为藻类对抗生素等新型污染物的响应机制提供理论依据.
全文HTML
-
研究使用的藻株拟柱孢藻(Raphidiopsis raciborskii)和四尾栅藻(Scenedesmus quadricauda)购自中国科学院(FACHB Collection;中国武汉). 在MA培养基[18]中培养,光暗比为12 h∶12 h,光照为50 μmol/(m2·s),温度(25±1) ℃,培养期内每天手动摇摆3~5次. 荧光显微镜下监测细菌污染比例不超过藻类生物量的1%[19]. 拟柱孢藻培养基中链霉素(Sigma,USA)处理组的最终质量浓度分别为0.05,0.1,0.2,0.5和1.0 mg/L,四尾栅藻培养基中链霉素处理组的最终质量浓度分别为1,2,5,10和20 mg/L,拟柱孢藻和四尾栅藻培养基对照组无链霉素处理,每组设置3个重复.
-
比生长率(μ)根据公式(1)计算.
式中,Ct2和Ct1分别是t2和t1时的藻密度[19].
培养96 h后,在8 000 rpm和4 ℃离心条件下收集所有藻类. 藻类样品放在5 mL,50 mmol/L磷酸缓冲溶液(pH=7.8)中保存. 丙二醛(MDA)含量、超氧化物歧化酶(SOD)和过氧化氢酶(CAT)的活性根据Bai等[19]的测定方法进行测定.
-
使用植物效率分析仪(PEA,Hansatech Instrument Ltd.,英国)测量叶绿素荧光. 进行叶绿素荧光测试前,将2 mL的藻液暗处理30分钟,然后利用植物效率分析仪进行分析. 叶绿素荧光诱导动力学曲线参考Kautsky效应,JIP-test参数由叶绿素荧光初始值计算获得[15-17, 20].
-
本研究的统计结果以平均值±标准方差表示,使用SPSS 16.0软件进行方差分析(ANOVA)和T检验,p<0.05为差异有统计学意义.
1.1. 培养条件
1.2. 比生长率、丙二醛含量和抗氧化酶活性
1.3. 叶绿素荧光诱导曲线和叶绿素荧光诱导动力学分析(JIP-test)
1.4. 数据分析
-
链霉素质量浓度为0.05,0.1,0.2,0.5和1 mg/L培养基中的拟柱孢藻的比生长率(μ)分别是对照组(0.00 mg/L)的0.75,0.64,0.34,0.33和0.25倍(p<0.05),详见图 1a. 链霉素质量浓度为1,2,5,10和20 mg/L培养基中的四尾栅藻的比生长率分别是对照组(0.00 mg/L)的1.19,1.10,0.66,0.63和0.51倍(p<0.05),质量浓度为1和2 mg/L的链霉素处理组中微藻比生长率明显较对照组高(p<0.05),详见图 1b. 拟柱孢藻和四尾栅藻处理组叶绿素a含量与链霉素质量浓度的变化特征与比生长率相似,详见图 1c和图 1d. 拟柱孢藻和四尾栅藻的EC50分别为(0.11±0.03)和(12.32±2.73) mg/L.
链霉素质量浓度为0.05,0.1,0.2,0.5和1 mg/L的培养基中,拟柱孢藻的丙二醛(MDA)含量分别是对照组的0.95,0.83,0.71,0.69和0.65倍,超氧化物歧化酶(SOD)活性分别是对照组的0.93,0.77,0.23,0.21和0.10倍,过氧化氢酶(CAT)活性分别是对照组的1.15,1.69,2.46,2.54和2.41倍(p<0.05),详见图 1e. 链霉素质量浓度为5,10和20 mg/L的处理组中,四尾栅藻的MDA含量、SOD和CAT活性发生了显著变化,明显与拟柱孢藻相异,详见图 1f. 当链霉素质量浓度为20 mg/L时,四尾栅藻的MDA含量和SOD活性明显升高,分别为对照组的2.81和2.24倍,而四尾栅藻的CAT活性显著下降,是对照组的0.76倍. 链霉素质量浓度为1 mg/L时,四尾栅藻的MDA含量、CAT和SOD活性与对照组相比变化不显著(p>0.05).
-
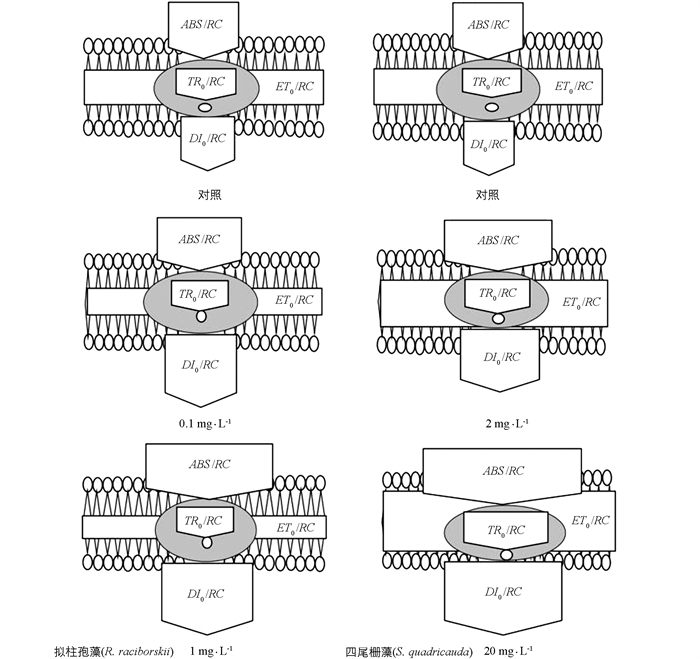
叶绿素荧光诱导动力学参数最大光化学效率(TR0/ABS=ΦP0,t=0,下同),光合系统Ⅱ活性反应中心捕获的激子驱动电子传递的比率(ET0/TR0=Ψ0),电子从QA-转移到电子传递链的量子效率(ET0/ABS=ΦE0) 和热耗散量子比率(ΦD0)变化特征见图 2a和2b. 拟柱孢藻和四尾栅藻中的ΦP0和ΦE0显著低于对照组(p<0.05),而ΦD0显著增加(p<0.05). 由图 2a和2b可知,拟柱孢藻中Ψ0增加,而四尾栅藻的Ψ0减少. 拟柱孢藻和四尾栅藻反应中心耗散的能量(DI0/RC)、单位光面积吸收的能量(ABS/CS0)和单位反应中心吸收的能量(ABS/RC)变化特征见图 2c和2d. 不同质量浓度链霉素处理组的DI0/RC,ABS/CS0和ABS/RC显著高于对照组(p<0.05). 链霉素质量浓度为1 mg/L的处理组培养96 h后,拟柱孢藻的DI0/RC,ABS/CS0和ABS/RC均出现增加,分别是对照组的3.36,1.24和2.63倍(p<0.05),20 mg/L链霉素的处理组中,四尾栅藻的DI0/RC,ABS/CS0和ABS/RC分别比对照组增加了54.02,7.03和16.24倍(p<0.05). 与对照组相比,不同质量浓度链霉素培养的拟柱孢藻的TR0/RC明显受到抑制,而不同质量浓度链霉素培养的四尾栅藻的TR0/RC均得到激活.
链霉素应激能量流动的管道模型如图 3所示,拟柱孢藻单位反应中心捕获的用于电子传递的能量(在t=0时)(ET0/RC)和单位反应中心捕获的用于还原QA的能量(TR0/RC)明显随链霉素质量浓度的增加而减少,DI0/RC和ABS/RC则随链霉素质量浓度的增加而增加. 四尾栅藻的ET0/RC,TR0/RC和ABS/RC在链霉素质量浓度较低(<2 mg/L)时显著增加,而DI0/RC在链霉素质量浓度较高时显著增加.
2.1. 比生长率、丙二醛含量、超氧化物歧化酶和过氧化氢酶活性
2.2. 光合系统Ⅱ的能量流动
-
水环境中抗生素的检出率较高,这与抗生素大量用于人类和动物的传染病治疗有关[14]. 有研究发现,抗生素对水体中的革兰氏阴性菌菌群、蓝藻的物种多样性和生物量有明显的抑制作用[21-24],这表明残留在水体中的抗生素能通过改变水体环境中的细菌、原生动物、藻类和浮游动物的数量破坏水体生态系统[25]. 本研究发现,不同质量浓度的链霉素均可抑制拟柱孢藻和四尾栅藻的比生长率(μ),链霉素质量浓度越高,抑制作用越明显,且蓝藻对链霉素的敏感性比绿藻更强,表明残留在水体中的低质量浓度抗生素对微藻及其生物量有抑制作用. 链霉素对念珠藻(Nostocales)的最小抑制质量浓度是0.28 mg/L,对斜生栅藻(Scenedesmus obliquus)的最小抑制质量浓度为21 mg/L[13],对铜绿微囊藻(Microcystis aeruginosa)的96 h EC50值为0.28 mg/L,对普通小球藻(Chlorella vulgaris)的96 h EC50值为20.08 mg/L[6]. 本研究发现,链霉素对拟柱孢藻的96 h EC50值为(0.11±0.03) mg/L,四尾栅藻为(12.32±2.73) mg/L,这与前人研究结果相近,表明较低质量浓度的链霉素能影响藻类种群的生长繁殖,且蓝藻较绿藻敏感. 另有研究发现,蓝藻的双分子脂膜系统缺少抗菌层细胞膜,而绿藻的双分子脂膜系统能减少链霉素向真核藻类的转运,因此,绿藻对抗生素的敏感性低于蓝藻[6].
藻类膜系统受到胁迫后会产生大量的活性氧(ROS),活性氧的增加会引起清除活性氧的超氧化物歧化酶(SOD)和过氧化氢酶(CAT)的活性增加,且活性氧的积累会导致丙二醛(MDA)含量上升[6, 26]. MDA被认为是生物对环境压力的反应产物[6]. 本研究发现,不同质量浓度链霉素培养条件下的拟柱孢藻MDA含量显著降低(p<0.05). 而暴露在链霉素环境中的四尾栅藻的MDA含量和SOD活性显著升高,CAT活性明显下降(p<0.05). SOD可以将超氧自由基转化为过氧化氢,过氧化氢可以被过氧化氢酶和过氧化物酶分解[27]. 显然,四尾栅藻的SOD活性增加、CAT活性降低有利于抵御ROS带来的氧化压力,这与前人的研究一致[28]. 本研究发现,低质量浓度链霉素处理拟柱孢藻时,MDA含量显著降低(p<0.05),表明链霉素暴露下没有引起拟柱孢藻膜脂的严重损伤. 链霉素质量浓度提高到5 mg/L时,四尾栅藻的MDA含量表现出显著升高(p<0.05),表明细胞因脂质过氧化受到的损害越来越严重. 主要原因可能是链霉素对原核与真核生物的影响机制不同,链霉素影响原核生物蛋白质的合成,干扰真核生物叶绿体蛋白质的合成,并导致胁迫后产生大量ROS[29].
-
光合色素吸收的太阳能在通过电子传递链时不断被损耗[30]. 本研究发现,与对照组相比,拟柱孢藻和四尾栅藻的ΦP0和ΦE0显著减少,ΦD0,DI0/RC,ABS/CS0和ABS/RC显著增加. 链霉素胁迫对光合系统Ⅱ的影响主要是维持能量同化和增强能量消耗[15, 31-32]. 拟柱孢藻和四尾栅藻的Ψ0和TR0/RC变化规律有明显差异,这表明四尾栅藻光合系统Ⅱ受体侧的电子传输效率较低,因为捕获能量而被大量耗散[32]. 因此,本研究认为,当拟柱孢藻暴露在链霉素环境时,从QA到QA-的电子传递是一个潜在的抑制靶点,容易受到影响. 相反,四尾栅藻的抑制靶点在QA-的下游,受影响较小. 有研究表明,不同质量浓度的污染物对藻类光合系统Ⅱ的影响与藻类QA到QA-的电子传递有较大的相关性,也可能与污染物暴露时间对藻类的发育和光合作用等的间接影响有关[33-35].
通过反应中心的基本能量通量合并到管道模型中的研究发现,ABS/RC和DI0/RC的相关性明显增强(图 3),这是因为拟柱孢藻和四尾栅藻光合系统Ⅱ对光能的接收能力发生了变化[36],本研究认为,主要原因是不同光合系统Ⅱ中单位反应中心的捕光复合物的数量发生了明显变化,这与前人的研究一致[37]. 与拟柱孢藻相比,四尾栅藻的优势在于它可以更好地以热能形式耗散电子传递链中的能量. 随着链霉素对这两个物种作用时间的增加,微藻的光合结构遭到破坏,单位面积上有活性的反应中心数量减少,光合系统Ⅱ电子传递受阻,抑制光合作用,导致微藻的生长受阻,这与前人的研究相近[38]. 主要原因可能是光合系统Ⅱ反应中心结构的改变,导致不参与电子传递的封闭反应中心数量增加,进而引起光合系统Ⅱ供体侧的电子传递被抑制[13, 39].
本研究认为,拟柱孢藻和四尾栅藻都暴露于链霉素环境时,它们的生长受到抑制,抗氧化机制和膜系统受到损害. 与四尾栅藻相比,拟柱孢藻对链霉素更敏感. 原核生物和真核生物在光合系统Ⅱ上的生理差异是引起拟柱孢藻和四尾栅藻对链霉素敏感性差异的主要原因. 深层原因是链霉素在光合系统Ⅱ中定位了不同的靶点,这可用于区分原核生物和真核生物光合作用的异质性. 本研究为进一步探讨链霉素等抗生素压力下自然生态系统中藻类群落的组成提供了理论依据.




 下载:
下载: