-
众所周知,将原药加工成制剂,是为了使农药活性成分能够到达靶标并作用于靶标,获得最大的生物活性.传统农药剂型在使用过程中,容易在靶标体或靶标附近的环境中被快速水解、光解或微生物细菌分解,使其浓度下降,防效降低,因此,传统农药经常需要多次喷洒才能保证其对病虫草害达到有效控制,但也造成了农药在环境中的大量累积,对环境造成较大影响.与此同时,多次施药也使得有害生物对药剂的抗性迅速提升.控制释放技术是指在一个特定的系统中,对特定的靶标,该系统内的活性物质可按照预先设定的浓度和时间持续释放,并达到预期的效果[1-3].控释技术广泛地应用于医药、化妆品、香料、染料、塑料、食品、显示材料及酶催化合成等行业和领域[4-5],农药制剂领域应用控制释放技术,可使得农药剂型能够定向控制释放,减少农药的施用次数,以达到高效、安全、经济和使用方便的目的.
关于以无机材料作为壁材包裹药物的研究中,Higashizaka E[6]使用可生物降解聚合物颗粒和陶瓷颗粒为壁材,对原药如4-异丙基环庚二烯酚酮进行包覆制备胶囊缓释剂,该研究提出,即便胶囊缓释剂暴露在阳光下抑或处于温度变化较大的环境中,均显现出被包裹的原药在外层可降解的颗粒降解后,才会通过含有陶瓷粒子的囊壁缓慢释放的结果. Qian K等[7]以氯化钙和碳酸钠为原料,采用反相微乳法对井冈霉素进行包裹得到了碳酸钙为囊壳的纳米胶囊,结果显示,所制备的纳米胶囊包封量较高,稳定性能好,具有明显的缓释效果.由此可以看出,无机材料作为一种微胶囊壁材,可以制备出一系列包封量高、稳定性好的微胶囊制剂.
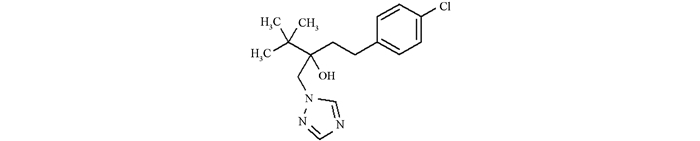
戊唑醇是拜耳公司研制的新一代高效三唑类内吸性杀菌剂,英文通用名:tebueonazole,化学名称:(RS)-1-(4-氯苯基)-4,4-二甲基-3-(IH-1,2,4-三唑-1-甲基)戊-3-醇,其化学结构如图 1所示.戊唑醇等三唑类杀菌剂具有其独特的杀菌作用机制,主要是通过破坏真菌的麦角甾醇的生物合成,从而阻碍真菌细胞壁的形成,最终起到防治真菌病害的作用[8-9].戊唑醇在使用过程中除了具备高效、低毒、广谱、用量小的特点外,还有增强根系的活性、调节植物的生长、延缓叶片衰老等优点,使其广泛地运用于作物的叶面喷洒或者种子处理当中,可有效防治禾谷类作物的多种白粉病、锈病、网斑病、赤霉病、根腐病、纹枯病、黑穗病等.因此,戊唑醇在农药杀菌剂领域有着广阔的发展前景.
然而,戊唑醇再生产使用中存在两个问题:第一,戊唑醇的主要剂型是乳油,持效期短,而且使用大量有机溶剂容易造成环境污染和资源浪费;第二,戊唑醇使用频繁以后产生的抗性问题.刘英华等[10]在研究禾谷丝核菌对戊唑醇的抗性中发现,多次施用戊唑醇使得菌系出现较高的抗性,且抗戊唑醇的菌系对三唑酮、丙环唑等药剂均产生高度的交互抗性,与敏感菌系相比,其交互抗性倍数分别高达31.2倍和22.8倍.
因此,为提高戊唑醇的使用效量、降低残留、减少环境污染,本文主要采用正硅酸乙酯(TEOS)和氯化钙为原料,形成以无机硅酸钙为壁材的新型戊唑醇纳米胶囊缓释剂,并对戊唑醇纳米胶囊缓释剂进行性能表征,并研究戊唑醇缓释剂释放规律.
HTML
-
97%戊唑醇原药,正硅酸乙酯,无水氯化钙,N,N-二甲基癸酰胺,农乳33#,司盘80等.
-
FA2004A型电子天平,上海精天电子仪器有限公司;SZCL-3A型数显智能控温磁力搅拌器,巩义市予华仪器有限公司等;BK系列生物显微镜,重庆奥特光学仪器有限公司;高效液相色谱仪(Agilent 1120),美国安捷伦公司;粒度分析仪(RISE-2006),济南润之科技有限公司.
-
通过复合凝聚法制备戊唑醇硅酸钙纳米胶囊缓释剂:将一定量戊唑醇溶解在适量的N,N-二甲基癸酰胺中,充分溶解后,依次加入乳化剂和正硅酸乙酯,充分搅拌混合均匀形成混合溶液A,然后将A溶液加入反应装置(装有电磁搅拌器、温度计、冷凝回流装置和滴液漏斗),高速(500 r/min)搅拌剪切10 min,形成透明油状的初乳.在室温条件下(25 ℃),将明胶溶液逐步滴加进入反应装置,戊唑醇在搅拌作用下形成O/W型小液滴,包裹在乳化剂胶束里面,同时硅酸根离子水解形成的SiO32-由于受分子间作用力(氢键、范德华力等)在胶束表面吸附,反应2 h,待正硅酸乙酯充分水解后滴加氯化钙溶液,使得Ca2+与SiO32-在乳化剂胶束表面反应沉淀、包裹、固化后形成戊唑醇纳米胶囊,反应完全后得到戊唑醇为囊芯、硅酸钙为囊壁的纳米胶囊.
通过调节加入正硅酸乙酯与氯化钙的量,制备不同芯壁比的戊唑醇纳米胶囊缓释剂.
-
通过电子显微镜对戊唑醇微胶囊的形态进行观察;通过SEM对其形态结构进行表征;通过马尔文激光粒度分布仪对戊唑醇微胶囊缓释剂胶囊的粒度分布进行评价.
-
高效液相色谱检测条件:以甲醇-水(体积比80:20) 为流动相,流速1.0 mL/min,柱温27 ℃,经Kromasil C18 250 mm×4.6 mm不锈钢色谱柱分离,可变波长紫外检测器223 nm检测.
称取一定量2.5%戊唑醇胶囊缓释剂样品,置于1 000 mL甲醇:水(20:80) 的混合液(作为释放的介质)中,以500 r/min搅拌,不同时间间隔下,定期取样,用0.22 μm有机滤膜过滤,通过HPLC分析戊唑醇的质量分数,同时在原来的溶液体系中补充加入释放介质,维持释放介质的体积恒定.戊唑醇的累积释放量为已溶出戊唑醇的质量与样品中戊唑醇的实际质量分数之比,绘制累积释放曲线.
分别向缓释介质中加入pH调节剂,调节缓释介质体系的pH,探讨不同pH条件下戊唑醇胶囊缓释剂的释放情况.同样,调节缓释介质体系的温度,研究不同温度条件下戊唑醇胶囊缓释剂的释放规律.
1.1. 材料与试剂
1.2. 实验仪器
1.3. 实验方法
1.3.1. 戊唑醇微胶囊缓释剂的制备方法
1.3.2. 理化性质的表征
1.3.3. 缓释动力学研究
-
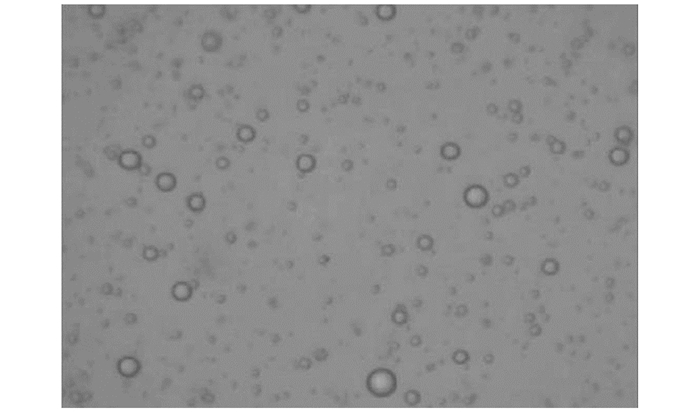
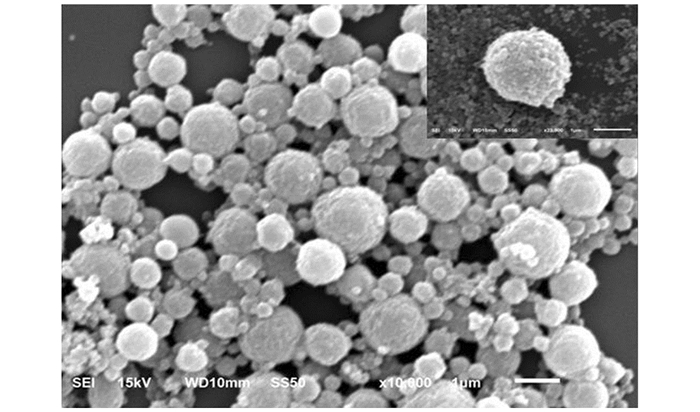
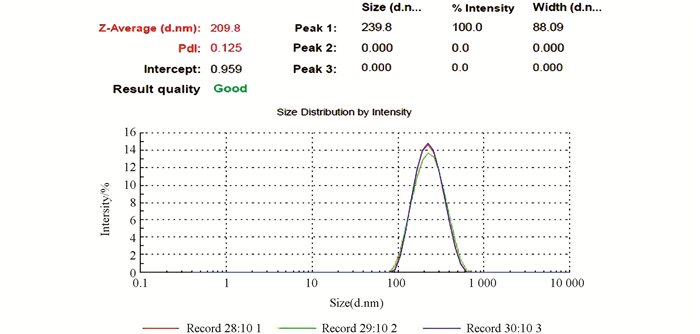
图 2,图 3和图 4中显示的是戊唑醇胶囊缓释剂的外貌形态和粒径分布情况,可以看出戊唑醇胶囊缓释剂的外貌形态为比较规则的球形,球体并不光滑,未出现较大的孔隙结构,有一定的交联.从图 4可以看出,平均粒径为209.8 nm,强度分布粒径为239.8 nm.
-
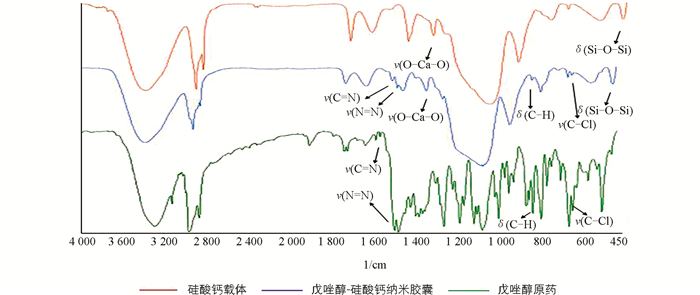
图 5显示戊唑醇胶囊缓释剂中主要物质的吸收峰,从图中可以看出,其中包含了戊唑醇和硅酸钙的主要红外特征吸收峰,可初步判断胶囊缓释剂中含有戊唑醇并生成了硅酸钙.
-
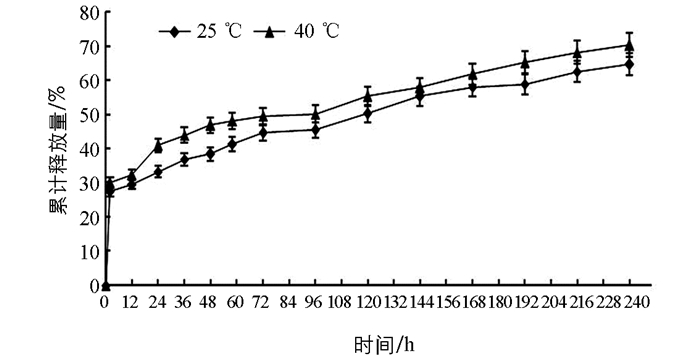
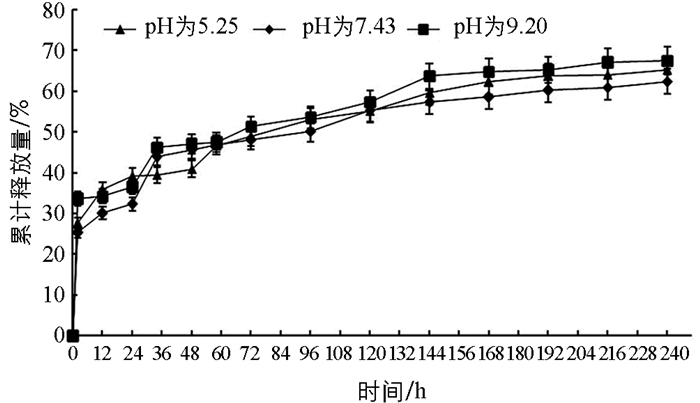
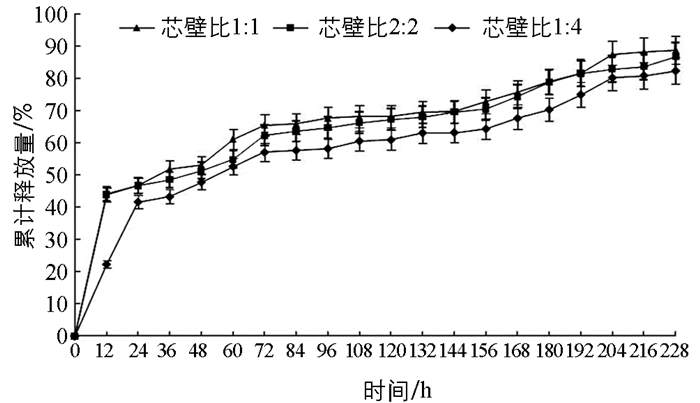
图 6表示在室内25 ℃和40 ℃时戊唑醇的累积释放量曲线,2.5%戊唑醇胶囊缓释剂中戊唑醇累积释放量随温度的升高而释放量递增. 图 7表示不同pH值(5.25,7.43和9.20) 时,2.5%戊唑醇胶囊缓释剂中戊唑醇的累积释放量曲线,表明在不同的pH条件下,戊唑醇胶囊缓释剂的累积释放量随着时间的增加而增加;pH为碱性时的释放量略高于pH为酸性和中性的释放量. 图 8表示不同芯壁比的纳米胶囊缓释剂的累积释放曲线,从图中可以看出,随着壁材增厚,戊唑醇纳米胶囊的释放速度越慢,这可能也跟壁材硅酸钙的抗机械强度能力相关. 图 6,图 7,图 8均可以看出,在24 h内,戊唑醇胶囊缓释剂的释放量为30%~40%,随着时间的延长,戊唑醇胶囊缓释剂的释放量逐步增加,最高可达到88%以上,表明戊唑醇胶囊缓释性能较好.
2.1. 戊唑醇微胶囊形态及其粒径分布
2.2. 戊唑醇胶囊缓释剂红外测定
2.3. 戊唑醇胶囊缓释剂缓释动力学研究
-
目前,戊唑醇制剂还主要是传统的乳油、可湿性粉剂等,针对现有制剂残留高、使用频量高及环境污染等问题,笔者研究了戊唑醇胶囊缓释剂的制备方法,同以往国内外戊唑醇相关研究比较,本研究采用正硅酸乙酯(TEOS)和氯化钙为原料,在制备过程中反应生成硅酸钙包裹固化戊唑醇,形成以无机材料硅酸钙为壁材的新型纳米胶囊缓释剂.同时,研究了不同温度、不同pH以及不同芯壁比对戊唑醇胶囊缓释剂释放性能的影响,摸清了戊唑醇胶囊缓释剂在上述条件下的释放规律,通过该缓释剂的制备有效地降低了戊唑醇的用量,节约了成本,降低了环境污染.
本研究还需要对戊唑醇胶囊的室内和田间药效进行验证,探讨在田间复杂环境下的释放机理及对作物的安全性,并研究胶囊在大田试验中对其释放的主要影响因子.






 DownLoad:
DownLoad: