-
近年来,肺炎克雷伯菌Klebsiella pneumonia,KP成为仅次于大肠杆菌的人类公共社区及医院感染最重要的条件致病菌[1-3].在动物方面,邹立扣等[4]对肺炎克雷伯菌引起猪的肺炎等作了相关报道,Aslan等[5]报道了肺炎克雷伯菌在引起牛的上呼吸道细菌性病原中占20%,许惠等[6]也报道了肺炎克雷伯氏菌引起犊牛发病率为25.0%,育成牛发病率为12.3%.近年来,肺炎克雷伯菌耐β-内酰胺类抗生素的报道日益增多,韦衍莉等[7]报道了氨苄西林的耐药率高达100%,白云等[8]报道了超广谱β-内酰胺酶(Extended-spectrum β-lactamases,ESBLs)阳性菌株耐药性明显高于阴性菌株,氨苄西林耐药率达100%,头孢菌素类抗生素有些也高达99%,即使是ESBLs阴性菌株,多重耐药的情况也十分严重. Thomson[9]、唐曙明等[10]报道了肺炎克雷伯菌超广谱β-内酰胺酶耐药基因主要与SHV,TEM,CTX-M有关.
本试验对分离自重庆荣昌和丰都牛场的12株牛源肺炎克雷伯菌进行了超广谱β-内酰胺环类药物敏感试验,通过PCR方法检测TEM,SHV,CTX-M基因,分析其所产生基因类型,为重庆市养牛场以超广谱β-内酰胺类抗生素进行肺炎克雷伯菌病的防治提供参考依据.
HTML
-
于2013年4月-9月在重庆(荣昌和丰都)两地区牛场采集并分离纯化、鉴定的肺炎克雷伯菌12株(荣昌地区7株,R1-R7;丰都地区5株,F1-F5).
-
普通琼脂(LB)培养基,药敏纸片购于杭州天和微生物试剂公司,常规质粒提取试剂盒E.Z.N.A. Plasmid Mini Kit I、Gold-view核酸染色剂(购于广州飞扬生物工程有限公司),Premix Ex Taq(Loading dye mix)购于大连宝生物公司(TaKaRa),DNA Marker DL2000、10×Binding Buffer(购于上海生工股份有限公司).
-
采用美国临床和实验室标准协会(Clinical and Laboratory Standards Institute,CLSI)推荐的K-B法将保存的12株菌株接种于LB液体培养基中,复苏菌种后接种到普通平板培养基,勾取单个菌落接种到LB液体培养基过夜培养12~18 h.每管菌液与标准比浊管进行比浊,用灭菌生理盐水稀释成0.5个麦氏比浊标准;用无菌棉拭蘸取菌液均匀涂布整个平板表面;室温下干燥后用无菌镊子取药敏纸片贴于平板表面,每张纸片的间距不小于24 mm,纸片的中心距平板边缘不小于15 mm,每个平板贴5~6张纸片,并将平板置于37 ℃恒温培养箱中倒置培养24 h,用标尺量取抑菌圈直径.
-
SHV,TEM,CTX-M基因引物设计参照文献[11],TEM基因:上游:5'-CAGCGGTAAGATCCTTGAGA-3',下游:5'-ACTCCCCGTCGTGTAGATAA-3';SHV基因:上游:5'-GGCCGCGTAGGCATGATAGA-3',下游:5'-CCCGGCGATTTGCTGATTTC-3';CTX-M基因:上游:5'-AACCGTCACGCTGTTGTTAG-3',下游:5'-TTGAGGCGTGGTGAAGTAAG-3'.
模版DNA制备及反应条件:按照试剂盒E.Z.N.A.Plasmid Mini Kit I操作步骤提取质粒DNA. PCR反应:PCR扩增采用25 μL体系. TEM基因反应参数为:95 ℃ 5 min;94 ℃ 30 s,52 ℃ 45 s,72 ℃ 45 s,循环30次;72 ℃ 7 min;SHV基因反应参数为:95 ℃ 5 min;94 ℃ 30 s,55 ℃ 1 min,72 ℃ 45 s,循环30次;72 ℃ 7 min;CTX-M基因反应参数为:95 ℃ 5 min;94 ℃ 30 s,57 ℃ 45 s,72 ℃ 45 s,循环30次;72 ℃ 7 min. 1%琼脂糖凝胶电泳,凝胶DNA成像仪分析结果.
1.1. 试验菌株
1.2. 材料
1.3. 药敏试验
1.4. PCR检测ESBLs菌株基因
-
12株肺炎克雷伯菌超广谱β-内酰胺酶类药物的耐药情况(表 1:耐药R、中介I、敏感S).从表 1中得知,阿莫西林和氨苄西林的耐药率为100%,头孢噻吩和先锋霉素Ⅴ的耐药率为17%,头孢曲松的耐药率为0.
-
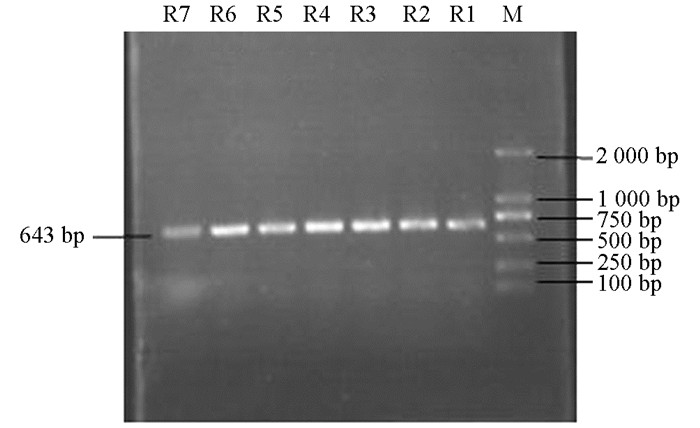
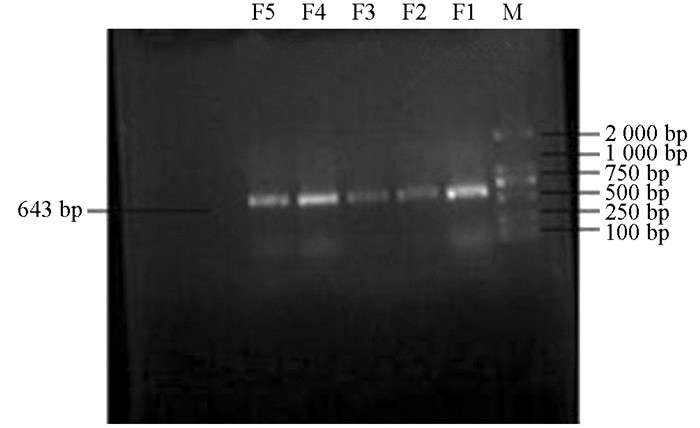
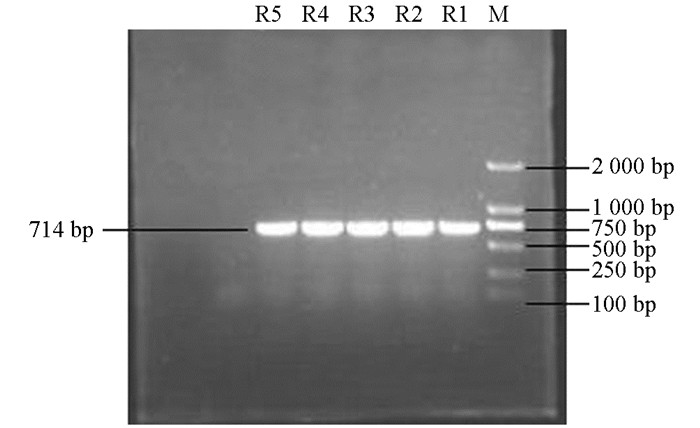
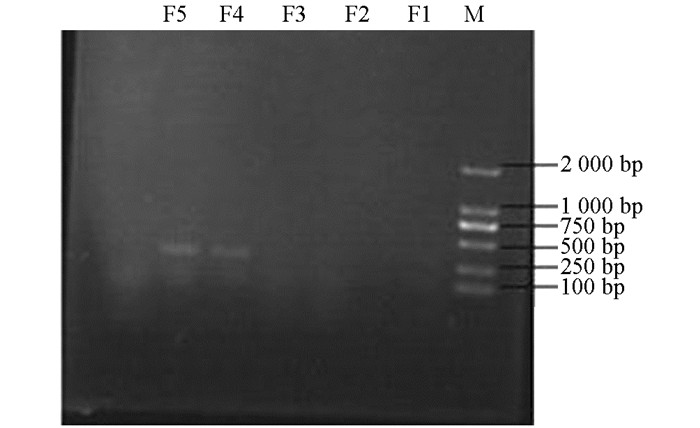
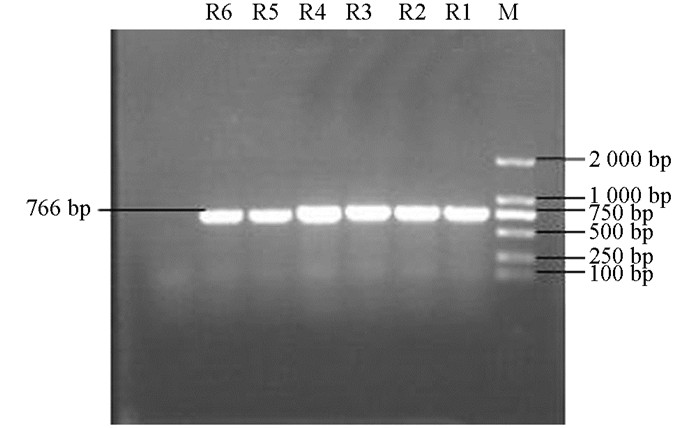
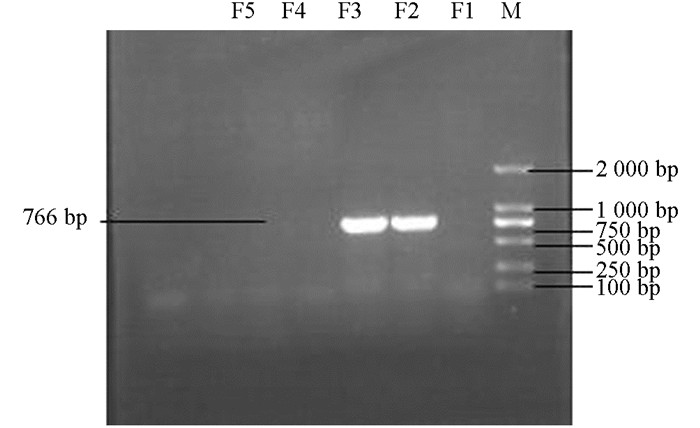
产ESBLs菌株3种耐药基因TEM,SHV,CTX-M的PCR扩增凝胶电泳结果如图 1、图 2、图 3、图 4、图 5、图 6所示.
-
从表 2可知,12株肺炎克雷伯菌均能检测出TEM基因,占100%;SHV基因共检测出5株(均来源于荣昌),占42%;CTX-M基因检测出8株(荣昌有6株,丰都有2株),占67%;携带有TEM和SHV两种耐药基因的共5株,占42%;TEM和CTX-M的共8株,占67%;SHV和CTX-M的共5株,占42%;3种基因均携带的共有5株,占42%.
2.1. 药敏试验结果
2.2. 产ESBLs菌株基因PCR扩增结果
2.2.1. 产ESBLs菌株基因PCR扩增凝胶电泳结果
2.2.2. 产ESBLs菌株基因检测结果
-
由于β-内酰胺类抗菌药物的广泛使用,使肺炎克雷伯菌产生超广谱β-内酰胺酶(ESBLs),因此对β-内酰胺类抗生素的耐药性日益严重.因编码β-内酰胺酶的基因多为质粒介导,使得耐药基因在菌株之间传播,致使耐药基因扩散蔓延[12].对肺炎克雷伯菌中ESBLs基因的检测,可了解ESBLs基因的分布特点.
在本研究中,两地区的肺炎克雷伯菌对β-内酰胺类抗生素均有比较严重的耐药性,对青霉素类抗生素耐药尤为严重,且表现为普遍多重耐药现象.药敏试验结果表明12株牛源肺炎克雷伯菌的阿莫西林和氨苄西林耐药率均为100%,与管中斌等[13]报道的基本一致,但头孢类药物有些许菌株耐药,头孢噻吩和先锋霉素Ⅴ的耐药率为17%,头孢曲松的耐药率为0,与其报道的有些差异.这可能与不同地区抗生素使用的种类和数量不同有关,也有可能与青霉素类抗生素价格相对低廉,临床生产中大量使用,而头孢类药物较昂贵,使用较少有关.
不同地区由于抗生素使用的种类和数量不同,所造成的抗生素选择性压力不同,相对应的ESBLs流行类型也有所不同[10, 14].本研究结果显示,12株来源不同的牛肺炎克雷伯菌中可检测出产生ESBLs耐药基因型有TEM,SHV,CTX-M3种,以TEM为主,其检出率高达100%,SHV的检出率为42%,CTX-M的检出率为67%;同一菌株携带多个基因的现象较为普遍,同时携带有TEM和SHV两种耐药基因的占42%;TEM和CTX-M的占67%;SHV和CTX-M的占42%;3种基因均携带的占42%,这与邹立扣等[4]报道的基本一致,与卢月梅等[14]报道的稍有差异. TEM是革兰阴性杆菌中最常见的β-内酰胺酶,主要介导细菌对各种青霉素和部分第1代头孢菌素耐药,而对第3代头孢菌素和酶抑制剂较敏感[15-16].本试验中分离的12株ESBLs肺炎克雷伯菌对氨苄西林和阿莫西林的耐药率为100%,而头孢曲松的耐药率为0,其原因可能是本地区以TEM流行为主. SHV,CTX-M两种基因型的检出率也很高,与TEM一起引起耐药.
肺炎克雷伯病原菌耐药性的产生与抗菌药物的使用不当有关,近年来随着抗生素的滥用,耐药菌通过质粒介导等方式,将耐药基因传递给牛体内的肺炎克雷伯菌群,或者直接感染,再将易感染的肺炎克雷伯菌排泄入环境中,如此恶性循环带来含耐药基因的菌株,给养殖业造成了巨大损失.
肺炎克雷伯菌主要经呼吸道及泌尿道感染,其药敏谱表现为多重耐药,尤其对青霉素类、头孢菌素类高度耐药.目前亚胺培南是治疗该菌最有效的药物[7-8],但主要用于人医方面.因此,在临床中要避免使用青霉素类,部分头孢菌素类(尤其是一、二代),可选用氨基糖苷类抗生素,如庆大霉素、卡那霉素、链霉素等药物,同时控制耐药菌株的散播和流行,做好饲养管理工作,监控细菌耐药性,合理使用抗生素.






 DownLoad:
DownLoad: