-
自信是优良的品格,也是文化生命力的心理基础[1-3].自信可以基于自身优势,表现为“我有”、“我能”,也可以基于自我定义或集体信念,表现为“我是”、“我们是”.自信复杂的内涵是基因、文化和社会共同塑造的结果[1, 3],个体自信品格受自我比较、社会比较以及情景定义的影响大[1, 4],而群体与文化自信更多反映了社会及文化事件、文化价值体系、社会结构及群体动员[1, 4].已有自信研究建立于行为观察基础上,有必要将更基础的学科证据(如基因和脑科学)整合到对自信的理解之中.
按内涵、塑造因素和社会适应方式的差别,自信可以区分2种风格:近通路和远通路.所谓近通路自信,是以迅速反应为特征、处理高威胁目标的自信.这种自信或基于进化或来自于自动化信念系统,支撑该自信的心理过程发动快、结构简单,效率高,对于环境挑战具有适应作用.儿童式争强好胜具有显著的近通路自信特性.对于人、社会、文化的复杂性而言,近通路自信不具有广泛适应性,其功能和表现必然随自我发展和时代变迁受到限定或修饰.例如,英雄时代“勇”的重要性[5]在进入封建社会后让位于“智”,有的文化则将关系性特质推到了最重要位置,孔孟学说即以“仁”为核心[6].概言之,生理性力量的价值在降低,智慧、美德的重要性在提升.工业革命之后,社会更是转换到了以能力为核心[1, 7],创造能力、组织能力、领导能力等不但成为人生赢家的核心素质,对于文化间竞争也具有决定性作用.基于这些素质、社会生活规则所发展起来的自信,出现得晚、形成过程长、心理过程复杂,可称为远通路自信.
远通路自信具有更复杂的内涵、表现形式和成长路径.就内容来看,信念和身体线索仍然是自信的重要因素,但更多源于适宜情景的内在自我优势:品德、智力、人际关系或这些品质的综合.就表现形式来看,与体能或信念具有绝对性不同,品德、智力、人际有关的素质是模糊的、相对的.就成长路径来看,内在认知框架和外在环境变化都会影响自信生成且可引发自信改变[1, 4],这个过程涉及自我、他人、群体,乃至于社会文化.即仅就认知自我而言,就需要协调自我认知(如自我比较)和社会认知(如社会比较),根据社会文化规范形成适应性、倾向性的自我判断.远通路自信的触发、形成和表达,往往伴随着缺少简洁应对模式的自我与环境不确定性,这需要更多认知参与,通过甄别梳理而后才能做出适应性自我判断和行为反应.
与2种具有不同内容、形式和功能的自信路径相对应,理论上也应有差异性神经生理过程作为其基础.从本质内涵和负担的功能来看,第一通路自信主要涉及本体自我与环境关系的确定性认知,在自我概念中具有基础性、自动化水平高的特点,其核心神经网络应该是以初级感知觉为起点,并以简洁方式连接到情绪运动脑区,以服务于快速反应.第二通路自信主要涉及自我认知过程,自传体记忆、自我参照加工、社会认知,这些自我概念和社会线索的加工都需要社会认知核心神经网络[8];此通路涉及的成分和过程复杂,推测前扣带回发挥监测作用,在需重评自我的情境下被激活,将注意力集中于自我相关的信息,背内侧前额叶则发挥评估作用对相关刺激进行评估,后侧扣带回发挥整合作用,整合自我相关信息[9],从而刷新自我认知.
对于揭示自信的神经生理过程和模式,静息状态下与自信功能有关的大脑区域自发活动特征为最基础的内容之一.静息态功能磁共振成像技术(resting state functional magnetic resonance imaging,rsfMRI)无需实验任务,操作简便,能够比较直接地探讨与行为表现相关的大脑自发活动[10].在rsfMRI中,有2个稳健指标可用于表达自信的神经基础,其一是反映大脑自发神经活动强度的自发低频波动(amplitude of low-frequency fluctuation,ALFF),其二是反映脑区自发神经信号同步性的静息态功能连接(resting-state functional connectivity,RSFC). ALFF是反映个体人格差异的有效指标[11-12],RSFC则可通过连通性、整合性良好的脑网络揭示外在行为表现的内在神经环路[13-14].
基于对自信和rsfMRI的理解,本研究采用ALFF和RSFC为指标描述静息状态下大脑活动与自信的特异性关系,探讨理论上的自信通路在大脑活动模式层面的特征,为理解自信形成和表达中基因-文化-社会的相互作用模式提供新证据,揭开自信研究的新篇章.
HTML
-
招募身心健康在校大学生42人,均为右利手、无精神病史且视力或矫正视力正常.数据预处理发现4人头动过大而剔除其数据,最终分析38人数据.其中,男21名,女17名,平均年龄(21.05±1.89) 岁.
-
总体自信问卷[15]共12个题项,采用从1(很不符合)到5(很符合)计分,总分越高代表自信水平越高.在本研究中,该问卷的克龙巴赫α系数为0.88;研究对象得分23~55分,平均分40.68,标准差7.47.
-
影像数据采集使用3.0T磁共振扫描仪(Siemens Medical Systems,Erlangen,Germany).高分辨率结构像由T1加权快速梯度回波序列(Magnetization Prepared Rapid Acquisition Gradient-echo,MPRAGE)取矢状位获得,其扫描参数如下:176层,重复时间为1 900 ms,回波时间为2.52 ms,扫描视野为256×256 mm2,翻转角为9°,层厚为1 mm,体素大小1×1×1 mm3.功能像由平面回波成像序列(echo planar imaging,EPI)获得,相关参数如下:重复时间为2 000 ms,回波时间为30 ms,图像矩阵为64×64,翻转角为90°,扫描视野为220×220 mm2,轴层为32,层厚为3 mm,体素大小为3×3×3 mm3.扫描过程中,要求被试闭上眼睛,但不要睡着,不要想特别的事情.扫描共采集242个时间点的全脑图像.
-
数据分析包含预处理、ALFF的获得、ALFF与自信相关分析,以及功能连接分析4部分.
-
数据预处理采用基于Matlab的Data Procssing Assistant for Resting State fMRI (DPARSF)[16].首先,在DPARSF中实现数据格式转化;为排除机器启动及被试适应过程对图像信号稳定性的干扰,剔除了功能像数据的前10个时间点,纳入最终分析有232个时间点.其次,进行时间层校正和头动校正以校正时间差和头动影响;然后将T1像分割为白质、灰质和脑脊液,为减少生理等因素影响,去除来自白质、灰质及脑脊液和24个头动参数等协变量[17-18];再将扫描得到的结构像配准到MNI(Montreal Neurological Institute)标准空间;随后采用半高全宽为4 mm的平滑核进行高斯平滑.最后对数据进行去线性漂移,从而去除低频漂移和高频噪声以增加信噪比.数据整体剔除标准为最大头动大于2 mm,转动大于2°.
-
在Matlab平台上调用Resting-State fMRI Data Analysis Tookit (REST,www.resting-fmri.sourceforge.net)进行计算.为将ALFF的计算限定在大脑灰质区域,此处采用了SPM8(www.fil.ion.ucl.ac.uk/spm)自带的灰质模板[19-20].其中,模板的阈值设为0.4,且被重采样为3×3×3 mm3.将每个体素(voxel)的时间序列进行傅里叶转换获得频域功率谱,然后将每个体素0.01~0.08 Hz功率谱平方根进行平均,就得到ALFF的值,最后以全脑为参照对其进行标准化[11].
-
在灰质模板基础上进行多重回归分析,探讨大脑局部自发神经活动与自信的关系.在SPM8中,以ALFF值为因变量,以自信得分为自变量进行多重回归分析.结果通过REST软件进行AlphaSim校正.校正阈限设为p<0.05,单个体素p<0.05,体素连接半径5 mm,团簇大小≥85体素(2 295 mm3).
-
为探究ALFF-自信相关分析得到的激活脑区之间及与脑内其他区域可能存在的功能网络而进行功能连接分析.将与自信显著相关的脑区作为种子点,在灰质模板上计算种子点与其他体素之间的功能连接.然后将生成的连接图计算功能连接强度与自信的相关,进行AlphaSim校正(阈值设为p<0.05),即得到与种子点有功能连接的脑区及功能连接强度与行为的相关系数.计算均在REST中完成,分析前对白质、脑脊液、全脑平均信号及头动参数这些无关变量进行了回归.
-
实验均得到西南大学心理学部伦理委员会批准.在实验前被试均签署知情同意书,功能磁共振扫描之后立即测量总体自信.实验结束后被试获得一定报酬.
1.1. 研究对象
1.2. 工具
1.2.1. 总体自信问卷
1.2.2. 功能磁共振成像系统
1.3. 数据处理
1.3.1. 数据预处理
1.3.2. ALFF的获得
1.3.3. ALFF与自信的相关分析
1.3.4. 功能连接分析
1.4. 程序
-
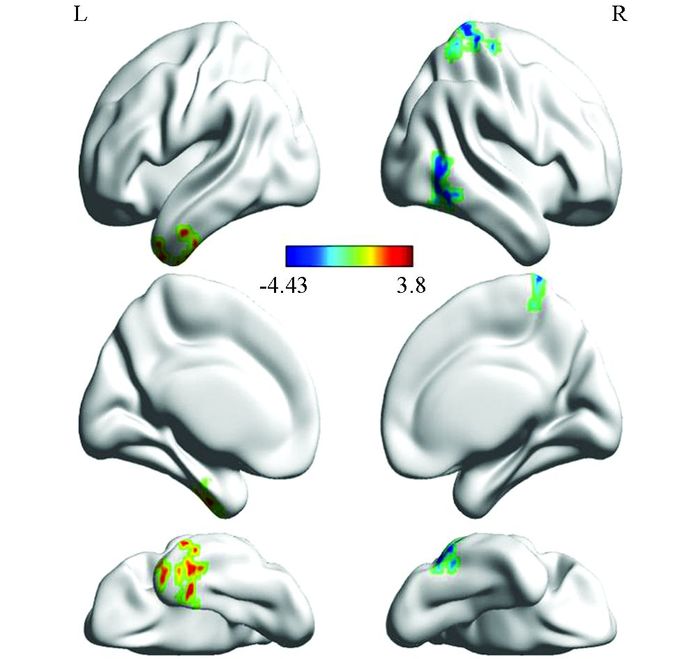
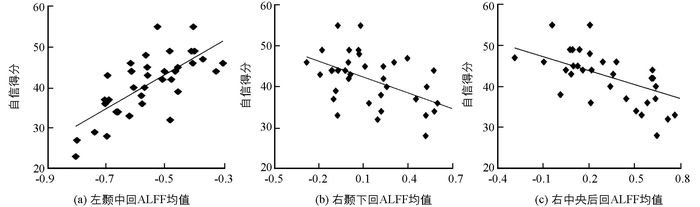
回归分析结果表明:左侧颞中回的ALFF值越大,自信水平越高(r=0.72,p<0.001);而右侧颞下回、右中央后回的ALFF值越小,自信水平越高(r=-0.68,p<0.001;r=-0.76,p<0.001)(表 1,图 1,图 2).
-
功能连接的分析结果显示,以左侧颞中回为种子点,未发现有脑区与此种子点的连接强度和自信相关.
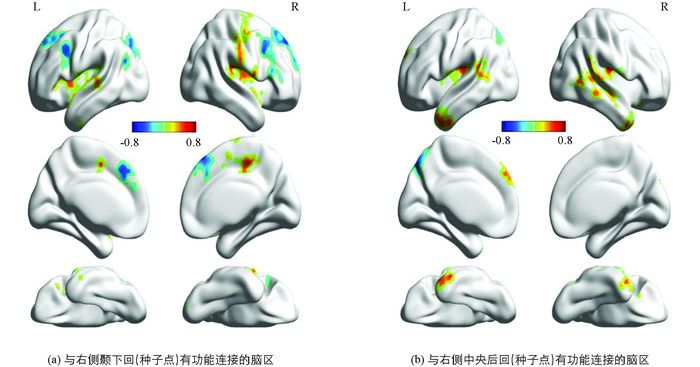
以右侧颞下回为种子点,右侧颞下回与左侧中央前回、右侧中央后回的连接强度和自信正相关;与双侧额中回、右侧额上回、左侧顶上小叶、左侧扣带回的连接强度和自信负相关.
以右侧中央后回为种子点,右侧中央后回与双侧脑岛、右侧颞中回、左侧颞上回、左侧额上回的连接强度和自信正相关;与左侧楔前叶的连接强度和自信负相关.结果见表 2、图 3.
2.1. 信有关的静息态活动脑区
2.2. 功能连接分析结果
-
本研究发现自信水平越高,左侧颞中回的自发神经活动越强.颞叶与视觉、听觉及语言、记忆等功能密切相关,颞叶中部不仅参与自我参照加工,还与记忆加工有关.颞中回是自传体记忆的重要脑区[21-22],个体对过去经历事件的记忆[23]的积极偏差[24]应是自信与该脑区自发活动正相关的基础.
右侧颞下回、中央后回的ALFF值与自信则是负相关,自信水平越高,这2个区域的自发神经活动越弱.颞下回是默认网络的重要节点,也是视觉腹侧通路的重要组成部分,主要参与面孔识别、有关物体识别的记忆等[25].中央后回是躯体感觉皮层的所在处,是脊髓与脑干的感觉传导通路[26],接受痛、温、触和本体感觉冲动并形成相应的感觉.这些信息对于衡量自我状态、自我与环境的关系至关重要.对于这些信息的整合易化程度越高,重组此类信息所需要的能量就较少,适应性就会越强.中央后回还与运动控制、协调有关[27].对行为的控制是应对挑战的重要成分,中央后回活动减弱意味着其资源的冗余,能够使应对有更好的准备,成为自信感来源的重要基础.
-
功能连接分析发现,以右侧颞下回为种子点,右侧颞下回与左侧中央前回、右侧中央后回、双侧额中回、右侧额上回等脑区活动的同步性与自信显著相关.颞下回、背内侧前额叶(额上回、额中回)、扣带回是默认网络的重要组成部分[28-31],内侧前额叶和扣带回则属于皮质中线结构,是默认网络的核心系统.默认网络参与维持大脑基线活动,与情景记忆提取、对周围环境和自我内省状态的监控以及持续进行的认知和情感过程有关[25],皮质中线结构则在自我参照加工中发挥核心作用[32].默认网络内的连接活动支持自信会包含自我参照加工.内侧前额叶还是社会认知网络的重要组成部分[33-35],参与社会信息加工[36-37],与社会认知功能网络的连接说明自信过程需要对自我有关的社会信息进行评估,这符合自信生成和表达需要协调自我认知和社会认知的理论判断.颞叶与中央后回脑活动同步的相关,则使自传体记忆与控制感、资源准备联系起来,这是自信者表现从容、进取的基础.
以右侧中央后回为种子点,右侧中央后回与双侧脑岛、右侧颞中回、左侧颞上回、左侧额上回的连接强度和自信正相关,与左侧楔前叶的连接强度和自信负相关.额上回、中央后回都具有控制功能,两者协同活动可提高个体的控制水平.脑岛具有情绪加工和处理能力,脑岛后部与积极刺激反应有关,比如金钱奖赏、愉快接触可激活脑岛[38].以上连接支持自信会伴随着积极的认知判断和情感体验.楔前叶与内在注意的导向、情景记忆检索以及自我相关加工有关[39].以往研究发现,抑郁症患者楔前叶的功能连接高于健康人群[40],可能与抑郁者冗思有关.自我反思对自信具有重要作用[41],然而过多自发反思也反映出强烈的自我不确定感[42],这会威胁自信,当敏感有余时就可能损害自信.
-
自信是进化的产物,又受社会和文化修饰,具有复杂的心理过程和表现形式,正是在这个判断基础上,本研究假设自信存在2种通路.从rsfMRI的结果来看,激活脑区和脑功能连接初步支持这个理论假设.其中,与近通路有关脑活动网路关联的功能包括:整合自我和外部信息实现对自我与环境状态的监控、认知与行为控制、以及奖赏.这些功能整合充分体现了近通路自信以自我为中心的控制感、能力感和积极色彩.与远通路有关脑活动网络关联功能包括:积极色彩的自传体记忆、社会认知过程、以及奖赏.这些脑功能整合则体现了自信受自我经验、社会过程甚至文化的影响.颞叶和中央后回及其相关的连接,可能更多反映了近通路自信的快速反应环路,与额上回、楔前叶等社会认知脑区的连接,更多反映了远通路自信在复杂的环境、自我、社会和文化影响下重新定位自我. 2个通路既有区别也有功能连接的重叠,例如都会联通到积极体验的脑区,以强化通路的适应性.
-
对于自信2个通路及其关系,当前研究仅提供了初步证据,未来可以从脑科学证据的多样性和自信的复杂性入手推进有关的研究.首先,脑成像研究可进一步提供结构态、任务态等方面多维证据.结构是功能的生理基础,特定功能频繁使用或强化训练也可带来大脑结构改变. 2种自信的群体差异应会在大脑结构(变化)上有所体现,典型群体的差异是一个可信赖的研究方向.
其次,进一步研究需要回答通路内部和通路之间的区别与联系.本研究中自信的操作性定义侧重于领域任务胜任感,并不明确区分自信的2种通路.就近通路自信的内容来看,有的来自于生理线索,有的则受自动化信念的制约. 2种通路还可能体现为“有”和“是”的区别,前者指依据“拥有”而定义自信,后者则强调自信来自于自我认定.另外,身体操控、本体感觉及行为控制更可能体现近通路自信,而自我反思和社会过程涉及多网络的协同,更多反映自信远通路.这些自信内容差异是通路结构与关系研究的切入点,对于确认和深化本研究发现也十分必要的,例如确定处于静息孤立状态的颞中回与其他脑区及脑网络的关系.
3.1. 与自信相关的脑区
3.2. 与自信相关的脑功能连接网络
3.3. 自信2种理论通路的静息态脑功能表达
3.4. 未来研究的方向
-
通过静息态功能磁共振成像技术,发现与自信有关的自发脑活动区为左侧颞中回、右侧颞下回和中央后回.通过功能连接分析发现自信与自传体记忆、社会认知、默认网络和奖赏系统等脑功能网络有联系.






 DownLoad:
DownLoad: