-
温度差异、季节交替及人为活动等因素常导致自然界水体的理化因子(如温度,溶氧)产生明显的时空异质性.水体理化性质的改变必然影响鱼类能量代谢强度,如标准代谢率(Standard metabolic rate,SMR)[1-4]. SMR是指变温动物在非活动、无食物消化吸收状态的最小机体能量消耗强度,是一种综合性的生理性能量消耗,通常以耗氧率表征SMR[5-6]. SMR是动物生活史理论重要的能量代谢参数,某些种类的SMR可占个体能量总支出的50%[7],并且个体在整个生活史中从外界获取的总能量有限,SMR所占比例将影响机体其他生理功能(如生长和繁殖)的能量分配,进而对个体乃至种群的生活史特征和适合度产生影响,相关研究备受关注[8-9].影响鱼类SMR的环境因素众多,如温度、溶氧、盐度和食物丰度等,以及易被研究人员忽略的因素如SMR的测定时间和流量,然而相关研究报道较为匮乏. SMR测定方法主要包括静水法和流水法,因后者具有测定便捷、可成批操作等优点被研究人员广泛采用,在此方法中流量成为鱼类SMR测定的重要影响因素[10].此外,现有研究基本上都采用溶氧测定仪测量水体溶氧水平并通过计算公式获得单尾鱼的耗氧率,但是所有测量仪器设备均存在固定的测量精度,据此推测较大流量可能导致连续测量过程中鱼类SMR数据变异程度更为明显.
中华倒刺鲃Spinibarbus sinensis是一种广温性底栖鲤科鱼类,也是我国长江上游水域的重要经济鱼类,喜成群栖息于底层多为乱石的流水中;该种鱼的生存温度范围为0~36 ℃,最适摄食生长温度为20~28 ℃,最佳摄食生长溶氧水平为3.9 mg/L以上[11-12].本研究以中华倒刺鲃幼鱼为实验对象,考察不同测定时段和不同流量对该种鱼SMR测定的影响,以确定鱼类SMR测定的适合时段和流量范围,为鱼类能量学研究提供理论技术支撑.
HTML
-
于重庆市北碚区人工养殖基地购买中华倒刺鲃幼鱼,购回后将鱼置于实验室循环水槽(1.2 m×0.55 m×0.55 m,约250 L)驯化1个月.驯化期间,用颗粒浮性饵料(中国通威公司)每3 d投喂1次,投喂前5 min关闭充气泵和驯化水槽的循环水泵,以减少环境扰动对实验鱼自由摄食的影响,投喂30 min后用虹吸管清除残饵和粪便以维持水质,随后重新开启充气泵和水槽的循环系统.实验用水为曝气3 d后的自来水,每3 d换水1次,每次约为驯养水体的三分之一,用充气泵不断向驯养水体充入空气使水体的溶氧水平大于7.0 mg/L,驯化水温控制在(25.0±0.5) ℃.
-
驯化结束后,随机选取鱼体健康、体质量相近[(6.32±0.12) g,n=80]个体作为实验对象,禁食24 h后对所有实验鱼进行SMR测定.本研究共设定5个流量梯度(1 L/h,2 L/h,3 L/h,4 L/h,5 L/h).其中,1 L/h和2 L/h的测量分别设定于第1 d的上午和下午(摄食后24~48 h),3 L/h和4 L/h的测量设定于第2 d的上午和下午(摄食后48~72 h),5 L/h的测量设定于第3 d的上午(超过摄食后72 h).在SMR测量时需提前将流量调整至目标流量梯度,每隔1 h测定1次,连续测定6个时间点;随后将流量调整至下一个流量梯度并间隔1 h,以减少流速调整对实验鱼生理状态产生的影响,再进行SMR测定.实验室流水式呼吸代谢仪每批最大样本量为40,故将实验鱼随机分成2批测定.
-
本研究采用流水式呼吸代谢仪进行中华倒刺鲃幼鱼SMR测定,每台代谢仪包括10个实验鱼呼吸室和1个空白呼吸室,共采用4台呼吸代谢仪.测定之前,将实验鱼装入代谢仪的呼吸室中驯化24 h(即禁食过程),同时测量体质量(精度为0.01 g)和体长(精度为0.1 cm).在SMR测定过程中,实验室环境保持安静以降低环境噪声对鱼生理代谢的影响. SMR mg/(kg·h)的计算公式为
式中,SMR为标准代谢率,ΔO2为空白呼吸室(无鱼)和实验呼吸室(有鱼)出水口的溶氧差值(mg/L),v为呼吸室的流量(L/h),m为实验鱼的体质量(kg).
本研究采用50 mL容量瓶测定目标流量,每一流量(1 L/h,2 L/h,3 L/h,4 L/h,5 L/h)的测量误差控制在±5.0%之内.经计算,上述梯度流量装满50 mL容量瓶的理论时间分别为180 s,90 s,60 s,45 s,36 s.代谢仪的水体温度控制在(25.0±0.5) ℃.
-
实验数据用Excel进行常规计算,所有数据均用“平均值±标准误”(Mean±SE)表示,显著性水平定为p<0.05.流量对中华倒刺鲃幼鱼ΔMO2(溶氧差值)变异系数的影响以及不同流量下SMR显著差异的比较均采用单因素方差分析(One-way ANOVA analysis).不同时间中华倒刺鲃幼鱼SMR的重复性采用Pearson相关分析.
1.1. 实验鱼来源及驯化
1.2. 实验设计
1.3. SMR的测定方法
1.4. 数据处理与统计
-
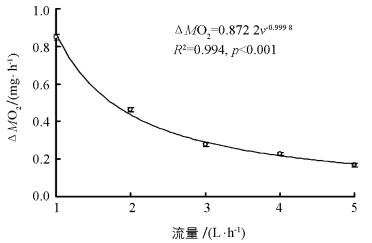
随着流量(v)逐渐增加,实验鱼呼吸室与空白对照的溶氧差值(ΔMO2)呈指数下降趋势,二者的拟合方程为ΔMO2=0.8722 v-0.999 8(图 1,R2=0.994,p<0.001,n=80).在拟合方程中,下降的指数(0.999 8) 接近1并且拟合度大于0.99,说明实验数据与理论数据十分接近.
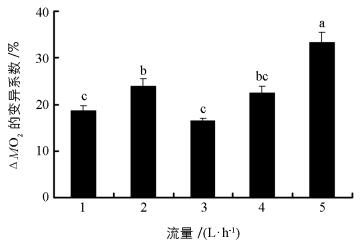
流量对中华倒刺鲃幼鱼ΔMO2变异系数产生显著影响(图 2,F=20.994,p<0.001).其中,在流量为5 L/h条件下,ΔMO2的变异系数最大,而流量1 L/h和3 L/h的ΔMO2变异系数最小.
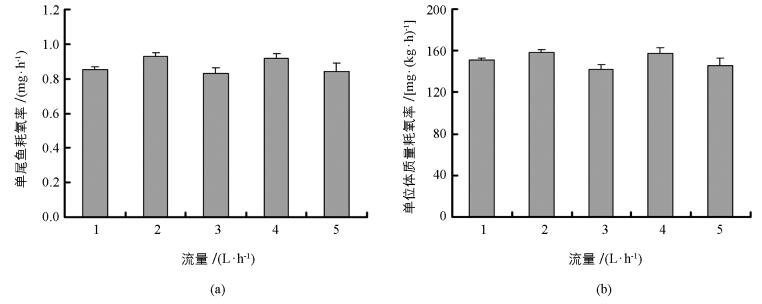
在不同流量条件下,中华倒刺鲃幼鱼的单尾鱼耗氧率和单位体质量耗氧率均无显著差异(图 3a,单尾鱼耗氧率为F=1.838,p=0.149;图 3b,单位体质量耗氧率:F=2.002,p=0.120).
-
用Pearson相关分析考察每一流量下不同时间之间的SMR相关性,在n=80时,当r>0.217,p<0.05时为显著;当r>0.283,p<0.01时为极显著(表 1).在流量为1 L/h条件下,SMR的重复性具有显著相关的比例为6.67%,呈极显著相关的比例为66.67%,不相关的比例为26.67%;在流量为2 L/h条件下,SMR的重复性具有显著相关的比例为20%,呈极显著相关的比例为66.67%,不相关的比例为13.33%;在流量为3 L/h条件下,SMR的重复性呈显著相关的比例为6.67%,呈极显著相关的比例为80%,不相关的比例为13.33%;在流量为4 L/h条件下,SMR的重复性呈显著相关的比例为6.67%,呈极显著相关的比例为60%,不相关的比例为33.33%;在流量为5 L/h条件下,SMR的重复性呈显著相关的比例为6.67%,呈极显著相关的比例为60%,不相关的比例为33.33%.这些数据表明当流量越大时,在不同测定时间下SMR重复性的比例趋向变小.
2.1. 流量与SMR及变异系数的关系
2.2. 5种流量不同时间下中华倒刺鲃幼鱼SMR的重复性
-
已有研究证明影响鱼类SMR的因素有很多,如温度[13-15]、溶氧量[16]等.流量作为重要的水动力学条件之一,对鱼类的生理、生存和繁殖有着极其重要的影响[17],它通过刺激鱼的感官使其产生不同的运动机制和相应的活动方式[18]. SMR是鱼类能量代谢模型中最敏感的变量之一,所有的环境因素在SMR中都有所反映[19].
本研究选取5个不同流量既考虑到较高流量可能带来数据变异较大的现象,也考虑到较小流量可能导致呼吸室中的实验鱼产生低氧胁迫,进而对测定正常生理状态下SMR产生影响.本研究发现设定的流量范围内中华倒刺鲃幼鱼SMR测定的平均值不受流量大小的影响.根据计算公式SMR=ΔO2×v/m,当流量逐渐增大,而溶氧差值(ΔO2)应逐渐减小,二者成反比关系.本研究发现,流量(v)与溶氧差值(ΔO2)拟合方程的R2为0.994(超过0.99),并且下降指数(0.999 8) 接近1.0,说明实测数据与理论数据十分接近(图 1),表明在1~5 L/h流量范围中流水式呼吸代谢仪空白管与实验管的溶氧差值与流量的关系符合数学理论模型.
用平均值衡量表型特征有助于比较不同处理间的差异,而反映组内数据变异则可用变异系数(CV=标准差/平均值×100) 评估.在上述公式中ΔO2=MO2空白管-MO2实验管,前者是指代谢仪空白呼吸室的溶氧水平,后者是装有实验鱼呼吸室的溶氧水平.本研究每一流量均设定了6个测量时间点,如果人为误差和仪器误差保持不变,那么不同时间条件下ΔO2也应保持不变.本研究通过CV评估ΔO2却发现,不同流量条件下ΔO2变异程度差异明显(图 2),并且较高流量条件下的ΔO2变异系数相对较大,这也支持本研究提出的猜测.另外,不论是单尾鱼耗氧率还是单位体质量耗氧率表征中华倒刺鲃幼鱼SMR时,不同流量条件下二者的平均值均不受影响,表明在1~5 L/h流量范围中以平均值表征中华倒刺鲃幼鱼SMR具有可靠性.进一步分析可知,本研究设定不同流量的测量时间介于中华倒刺鲃幼鱼最后1次摄食后的时间范围内(24~72 h及以上),并且不同时间测量中华倒刺鲃幼鱼的SMR无显著差异.现有研究认为鱼类SMR测量至少在摄食后24 h以上[6, 10].然而,过长时间的禁食会导致鱼类正常生理状态进入饥饿状态[20].结合本研究的实验结果,建议鱼类SMR的测量时间范围介于末次摄食后24~72 h.
有关SMR测量过往研究所采用的流量不尽相同(表 2),如鲫Carassius auratus幼鱼的流量范围介于4.8~6.0 L/h之间,鳟Salmo trutta的流量范围介于1.47~1.68 L/h之间,大西洋鲑Salmo salar的流量为2.1 L/h.本研究设定的流量范围介于1.0~5.0 L/h之间,涵盖上述研究的测量范围.值得注意的是,这些研究实验鱼的体质量与流量呈一定程度的正相关,即体质量越大的鱼需要的SMR流量也相应越高,与研究者考虑到流水式测量过程中实验鱼呼吸导致呼吸室水体溶氧水平降低,但降低的溶氧水平不成为鱼的低氧胁迫有关,提示相关研究需要关注ΔO2的实际大小与不同鱼类低氧耐受能力的关系.
表型特征的稳定性是指在不同时空或环境条件下,实验动物的可量表型性状测量结果在数值上的大小排序问题,可通过相关性分析评价排序结果的优劣[23],即相关数值r值越高表示表型特征的稳定性越好[24-25].已有研究证明不同鱼类在不同时期、不同生活状态下具有相应的SMR[24, 26],但在一定时间内,SMR将趋于稳定[23].本研究通过考察不同流量下中华倒刺鲃幼鱼SMR相关性分析发现,随着流量的加快,实验鱼SMR重复性呈现出显著和极显著的比例逐渐减少,而呈现出不相关的数据比例逐渐增大,表明流量越大,SMR稳定性逐渐降低.
不同流量条件下中华倒刺鲃幼鱼ΔO2的变异系数差异明显;流量对中华倒刺鲃幼鱼的SMR测定结果无显著影响,然而当流量越大时在不同测定时间下SMR重复性的比例趋于变小.本研究建议鱼类幼鱼SMR测定的流量不宜过快,并且SMR测定时间介于末次摄食后24~72 h之间.






 DownLoad:
DownLoad: