-
功能性官能团取代的有机羧酸配体和金属配位组装合成的金属羧酸配位化合物,作为一类新兴多孔材料,因其在能源气体吸附分离、不对称催化、磁学、荧光、化学传感等众多领域有潜在应用,其设计合成、晶体结构和性质研究成为国际国内研究热点[1-5].然而,研究人员报道合成的大多数化合物在接受长时间X射线照射后结构坍塌而无法分析其晶体结构,还有一部分材料在离开母液或客体分子交换脱除过程中主体骨架结构坍塌,导致此类多孔材料在众多应用研究领域受限.
Yaghi O M,Eddaoudi M,Liu Y L等研究人员通过选用四配位的金属中心和咪唑羧酸及其衍生物类配体合成具有类分子筛拓扑结构的ZMOFs系列配位化合物,并开展了其在气体吸附分离领域的应用研究[6-8]. Cohen S M研究小组发表综述论文,总结了后合成策略及功能性官能团的修饰改善配位化合物的孔道结构内表面的化学性质,拓展其在立体化学催化、药物担载与缓释等领域的潜在应用[9-10].如何提高合成晶体材料骨架的热稳定性和化学稳定性,拓展其应用研究领域,现有文献主要报道了3种合成策略:①使用咪唑和咪唑羧酸衍生物类配体合成具有类分子筛拓扑骨架结构的化合物[11-12];②后合成修饰嫁接一些功能性的官能团改进孔道内表面结构;③设计合成多核金属中心的次级结构单元提高材料的稳定性[13-15].
本研究选取吡啶基取代的均苯二羧酸衍生物配体和过渡金属锌在混合溶剂热体系中组装合成了一例具有开放骨架结构的双核锌羧酸配合物——Zn2(C21H9NO8)(H2O)(配合物1),并完成了配合物1晶体结构解析和相关表征工作.
HTML
-
配合物1的单晶结构使用德国Bruker Apex Ⅱ QUAZR CCD单晶衍射仪收集衍射数据,结构表征使用Rigaku D/MAX-2550型X-射线衍射仪测试.化合物元素分析采用Perkin-Elmer 3300DV ICP和elementer vario MICRO元素分析仪.红外光谱使用Bruker IFS-66V/S光谱仪抽真空测试.
-
1,3-di(3',5'-dicarboxylphenyl)pyridine配体(C21H13NO8,H4L) (0.1 mmol),Zn(NO3)3·6H2O (0.2 mmol),N,N-二甲基乙酰胺(CH3CON(CH3)2,DMA),CH3CN和H2O(体积比为1.0:0.50:0.25)加入到20 mL的玻璃小瓶中,超声混合均匀,用锡纸密封后拧紧瓶盖,放在85 ℃的烘箱内反应20 h得无色透明四棱柱形状的配合物1的单晶体.
1.1. 仪器与试剂
1.2. 配合物1的合成
-
配合物1的晶体使用Bruker Apex Ⅱ QUAZR CCD单晶衍射仪收集衍射数据.在温度为(23±2) ℃的条件下用石墨单色器单色化的CuKα射线(λ=0.071 073 nm),ω扫描方式收集数据.配合物1在2.33<θ<24.31范围内共收集到衍射点3 398个,其中独立衍射点为2 600个(Rint=0.047 2).运行Process-auto程序还原衍射数据,使用SHELXTL-97程序对配合物1的晶体结构进行解析和精修.结构解析和精修分别采用重原子法和全矩阵最小二乘法.首先从差傅立叶图中确定Zn原子衍射峰位置,再从残峰中确定咪唑羧酸和草酸配体中O,N和C原子衍射峰位置,最后对所有原子做各项异性精修.详细晶体学数据参看表 1.
-
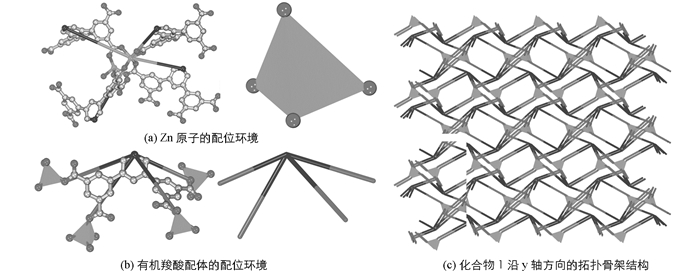
单晶X-射线衍射分析结果表明配合物1结晶在正交晶系,P212121 (No.19),化学式为Zn2(C21H9NO8)(H2O).配合物1的不对称结构单元中,包含2个晶体学独立的Zn原子、1个H4L配体、1个配位H2O分子. Zn1原子分别和4个有机羧酸配体结构中的羧基氧原子O2,O3,O6,O8单齿配位,Zn2原子分别和3个有机羧酸配体和一个配位水分子结构中的O4,O5,O7和O9配位,2个Zn原子均形成四面体的配位构型.由于2个配体结构中羧基氧原子的螯合配位作用,使得金属Zn和4个配体羧基氧原子螯合配位,形成1个双核锌的次级结构单元,在拓扑结构分析中可以抽象成一个4-连接四面体构型的拓扑学节点(图 1(a)).每个有机羧酸配体通过结构中的4个羧基氧原子分别和4个双核锌的次级结构单元配位连接,由于配体结构中2个苯环扭曲形成的两面角,因此整个配体可以抽象为一个非平面结构的4-连接拓扑学节点(图 1(b)).配合物1的拓扑结构见图 1(c).
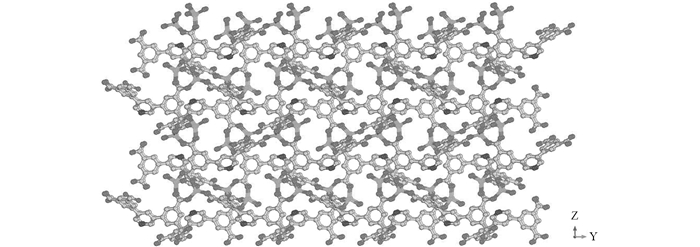
配合物1的三维结构见图 2,过渡金属Zn通过有机羧酸配体结构中羧基氧原子的配位连接作用形成配合物1的三维开放骨架结构,配合物1沿y轴方向可以分别观察到0.646 7 nm×0.486 6 nm和1.020 9 nm×0.210 7 nm两种不同孔径尺寸的1D螺旋孔道结构. PLATON软件计算结果显示,配合物1的每个单胞中,溶剂分子可占有体积为1.993 6 nm3,孔隙率高达54.5%.双核金属锌的次级结构单元稳定了配合物1的晶体学骨架结构,为其在能源气体吸附及分离等领域的潜在应用性质开发提供了可能性.
2.1. 结构测试
2.2. 结构描述
-
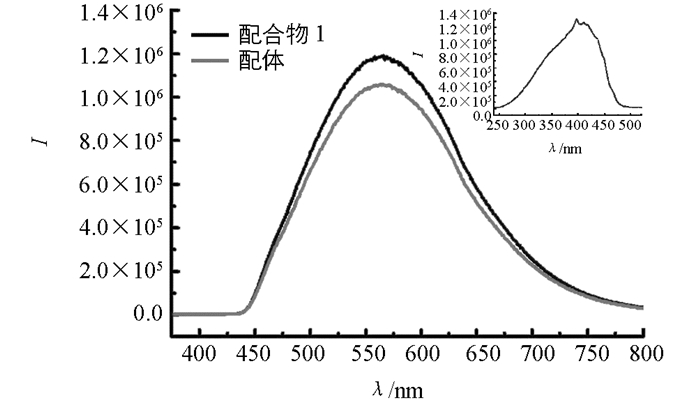
配合物1的荧光测试结果见图 4,选用410 nm波长激发,在375~800 nm波长范围内扫描有机配体H4L和配合物1的荧光发射光谱.对有机配体激发扫描测试结果在563 nm处出现一个较强的宽的荧光发射峰;配合物1在555 nm处出现类似的荧光发射峰.配合物1的荧光发射强度增加且主峰位置相对于配体出现少许蓝移,可归属为配体的去质子化过程以及金属配位效应降低了HOMO-LUMO能隙;两者荧光发射峰形状类似,可见配合物1的荧光性质是由配体结构内部发生的π*→π电子跃迁所致.
-
在DMA/CH3CN/H2O的混合溶剂热体系中,本研究使用有基羧酸配体1,3-di(3',5'-dicarboxylphenyl)pyridine (C21H13NO8,H4L,)与过渡金属锌配位合成了一例具有三维开放骨架的配位化合物1——Zn2(C21H9NO8)(H2O).单晶X-射线单晶衍射分析结果显示有机羧酸配位通过结构中的羧基氧原子和锌原子配位形成了1个双核锌的次级结构单元,每个有机配位作为1个4-连接的拓扑学节点在3个方向上的配位连接形成配合物1的三维开放骨架结构,沿y轴方向可观察到一维孔道结构.双核金属锌的次级结构单元稳定了配合物的主体骨架,有益于进一步开发其在气体吸附等领域的潜在应用研究.






 DownLoad:
DownLoad: