-
汞是毒性较强的重金属离子之一,对水生生物和人类健康有很大的危害[1-2].汞中毒主要损坏人及动物的神经系统,破坏脑组织,此外对人的消化系统、听力和皮肤等都有巨大影响.重金属离子的检测方法有很多,常用的方法有电感耦合等离子体发射法[3-4]、冷蒸气原子荧光光谱法、原子吸收光谱法[5-7]和氢化物发生原子荧光光谱法等[8].近年来又出现了很多检测汞离子的新方法,如以各种生物化学物质:荧光物质[9-10]、金纳米粒子[11]、有机分子[12]、DNA酶[13]和电化学等[14]为基础的传感器方法,这些方法灵敏度较高,但有些方法存在样品预处理复杂、仪器昂贵、费时和易受其他金属离子影响等不足.
胶体金免疫层析技术(ICA)具有方便快捷、不需要任何仪器和设备、肉眼即可观察、可以用于现场临时检测、成本较低、操作简单等优点[15].近年来,不同重金属离子的胶体金免疫层析试纸条技术已有报道,如镉离子[16]、铅离子[17]、铬离子[18]、铜离子[19]和汞离子[20-21],但是由于大部分的金属离子抗体都是针对金属离子-配体的复合物,而不是单独的金属离子,所以在检测中必须预处理样品.本课题组已经成功制备出了针对单独汞离子的单克隆抗体,并且应用于酶联免疫吸附分析方法[22]、表面增强拉曼光谱[23]及电化学发光[24]等研究,结果良好.本研究利用该单克隆抗体针对单独汞离子的强特异性优点,建立了一种快速直接检测汞离子的胶体金免疫层析试纸条方法,该方法快速准确,不需要繁琐的样品预处理过程.
HTML
-
氯化汞、牛血清蛋白、卵清蛋白、兔抗鼠IgG购于sigma公司;氯金酸、柠檬酸钠购于上海国药集团有限公司,硝酸纤维素膜、PVC底板等购于上海杰一生物科技有限公司;Hydroflex洗板机和infinite M200PRO酶联免疫检测仪购自奥地利TECAN公司;紫外-可见分光光度计购自美国PE公司.
-
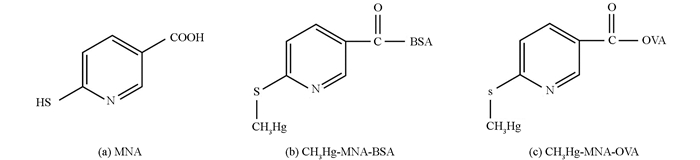
本研究选用了一种双功能试剂——6-巯基烟酸(MNA,图 1(a)),由于其结构中既有巯基又有羧基,所以既可以与汞连接,也可以通过活化酯法与蛋白连接.参照先前的研究[22],制备出CH3Hg-MNA-bovine serum albumin (BSA) (图 1(b))作为免疫原,CH3Hg-MNA-ovalbumin (OVA) (图 1(c))作为检测抗原.然后通过细胞融合杂交瘤技术制备出了针对单独汞离子的特异性单克隆抗体.
-
首先参照LI等[25]的方法制备出胶体金,然后将胶体金溶液用0.1 mol/L K2CO3调节pH值到8.2,加入适当体积的汞离子抗体,室温1 h,然后加入5%的BSA封闭30 min.采用低温超速离心法纯化后,收集管底红色流动液体,加入8%的蔗糖水溶液混悬,4 ℃保存备用.
-
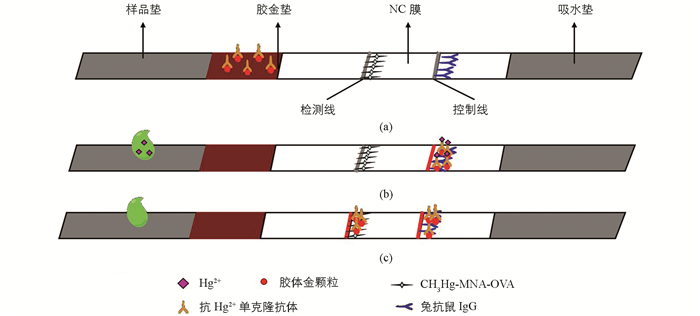
试纸条由样品垫、胶金垫、NC膜、吸水垫和PVC底板组成,其组装顺序见图 2(a).先将NC膜剪切成合适的尺寸,将包被抗原和二抗线划好,干燥,然后贴在PVC底板相应的位置;接下来是贴胶金垫,将制备好的胶金垫上端与NC膜下端相连,并重叠1 mm左右;将样品垫与胶金垫的下端相连,重叠1 mm左右;然后贴吸水垫,吸水垫与NC膜上端连接,重叠1.5 mm左右;最后将制备好的试纸条切成约4 mm宽的细条,密封干燥保存备用.
1.1. 试剂与仪器
1.2. 抗原的合成及抗体的制备
1.3. 胶体金标记抗体的制备
1.4. 试纸条的组装
-
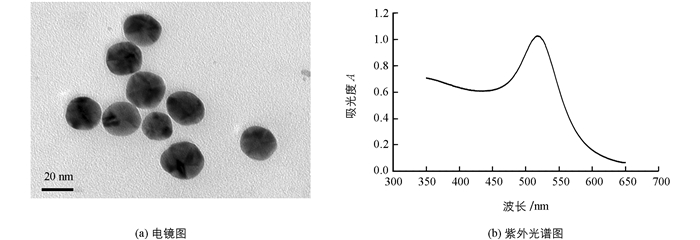
本实验制备了19 nm和40 nm的胶体金,在优化实验条件中发现,40 nm胶体金对抗体要求量太大,试纸条的显色情况也不理想,所以选择19 nm的胶体金为实验所用.本研究制备的胶体金,从肉眼观察是呈透明的酒红色,无浑浊感,初步证明胶体金质量较好.在透射电镜下观察显示(图 3(a)),颗粒大小基本一致,形状均匀.从紫外扫描的图谱看出(图 3(b)),制备的胶体金紫外最大吸收波长为520 nm,根据线性回归方程得到胶体金颗粒的直径约为19 nm,且峰宽较小,说明颗粒均匀.
-
由于要检测的是金属离子,分子质量很小,所以试纸条采用竞争法.当将试纸条插入待测溶液中时,样品溶液通过毛细管作用移动到胶金垫,若溶液中含有足够多的汞离子时,就与金标抗体完全结合,一起到达硝酸纤维素膜,不与膜上检测线的包被抗原结合,T线就没有颜色,复合物继续向前与二抗结合,C线出现红色,也就是说若待测样品是阳性的,则只在C线处出现一条红色条带(图 2(b));相反,若是阴性样品,则金标抗体与膜上的包被抗原结合,最后出现两个红色的条带(图 2(c)).若两条线均未出现或者只出现检测线,则说明试纸条失效.
经过实验条件的筛选,试纸条的最佳条件如下:包被抗原(CH3Hg-MNA-OVA)浓度为0.25 g/L,胶体金标记抗体的最佳质量浓度为25 mg/L.
-
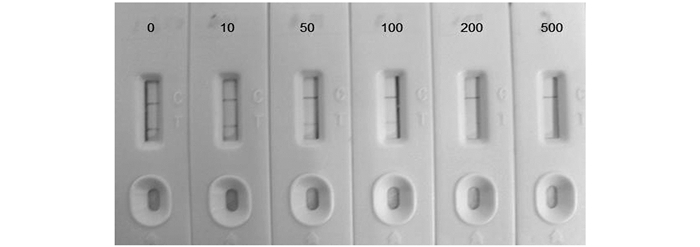
试纸条的灵敏度用检出限来表示,当检测线和质控线都是红色条带时,样本为阴性,当检测线为无色时,样本为阳性.也就是说,随着标准品质量浓度的增大,当达到一个极限值时,检测线颜色消失,这时的浓度就是检出限.本实验将汞离子标液稀释成一系列的质量浓度0,10,50,100,200,500 μg/L,取200 μL进行实验,观察结果,确定检出限,从图 4可以看出,随着汞离子质量浓度的增加,检测线条带的颜色逐渐减弱,当质量浓度为50 μg/L时,检测线颜色明显变弱,当质量浓度增加到100 μg/L时,检测线没有颜色,继续增大汞离子的质量浓度,检测线都不显色,所以判定检出限为100 μg/L.
同时,为了考察试纸条的特异性,选取了相关的物质做交叉反应实验,分别有Co2+,Mn2+,Ni2+,Fe2+,Pb2+,Cd2+,Zn2+和Sn2+,每种离子配制质量浓度为1 000 μg/L,然后用组装好的试纸条检测,结果发现,各种离子的检测线均有颜色出现,说明无交叉反应,抗体对汞离子特异性良好.
-
为了验证方法的准确度,对样品进行了加标回收实验,汞离子加标质量浓度为0,20,50,100 μg/L,用ELISA方法和试纸条分别测定,将结果进行比较,二者的结果显示见表 1,在ELISA的线性范围内,二者结果一致,说明建立的试纸条方法可以测定水样中的汞离子.
2.1. 胶体金的选择
2.2. 试纸条的结果判定及条件优化
2.3. 试纸条的灵敏度和特异性
2.4. 试剂条与ELISA的对比
-
本研究主要建立了检测汞离子的胶体金免疫试纸条方法,运用本实验室制备的抗汞离子的单克隆抗体,成功得到了金标抗体作为检测探针,并通过各种实验条件的优化,建立了快速、简单、可靠的检测水样中汞离子的免疫层析试纸条技术.该方法样品处理简单,无需繁琐的仪器操作,准确度高,特异性强,检测结果只需要十几分钟,适合用于现场操作,大大提高了检测效率.






 DownLoad:
DownLoad: