-
类柠檬苦素(limonoids)是一类高度氧化的四环三萜类植物次生代谢物质,广泛存在于芸香科(Rutaceae)和楝科(Meliaceae)植物中,通常以糖苷和苷元两种形式存在[1-2].芸香科柑橘属(Citrus)植物的种子被认为是天然类柠檬苦素的重要来源[3].类柠檬苦素生物合成途径存在于几乎所有柑橘品种中,诺米林、奥巴叩酮和柠檬苦素是其中最主要的3种[4].研究表明类柠檬苦素具有抗癌[5]、抗氧化[6]、抗病毒[7]、抑制HIV[8]和杀虫[9]等多种生物活性.而另一方面,作为柑橘产品开发利用过程中最主要的两种苦味物质之一,由柠檬苦素苷元引起的苦味是柑橘产业中一个巨大难题[4, 10].
截至目前,已从柑橘中分离出大约39种柠檬苦素苷元和17种糖苷[11-12].薄层色谱法(TLC)[13]和高效液相色谱法(HPLC)[14]都可以用于类柠檬苦素的分析检测.其中,HPLC是类柠檬苦素分离和鉴定中最常用的方法[15].随着分析技术的发展,超高效液相色谱-四级杆飞行时间质谱联用技术(UPLC-Q-TOF-MS)在柑橘化学成分分析中的应用越来越普遍[16-18].与HPLC比较,UPLC在速度、灵敏度和分离效率上表现出明显的优势[19-21].此外,新型Q-TOF-MS为高分辨的质谱,可提供更准确的离子碎片信息,能快速准确地对天然产物中一些化合物进行定性分析[22].
已有文献报道柠檬苦素和奥巴叩酮的MS/MS详细的质谱裂解途径[23],目前诺米林的质谱裂解途径尚未解决.而诺米林作为合成其他所有柑橘中类柠檬苦素化合物的前体物质,其研究显得尤为重要.此外,类柠檬苦素在芸香科和楝科植物中可作为一类特殊的分类学标记物[24].因为特征性的类柠檬苦素及其生物合成途径只在特定的种或属中存在.因此,在同一分析方法下对不同品种柑橘种子中类柠檬苦素含量和分布的研究具有重要意义.已报道的分析方法存在速度慢、重现性差、操作复杂和灵敏度低等问题.目前类柠檬苦素化合物在柑橘中的含量尚无定论,各实验室报道有所不同,相应的定性定量检测方法及商业标准不完善.
本研究拟通过UPLC-Q-TOF-MS对柑橘种子中柠檬苦素、诺米林和奥巴叩酮进行定性定量分析,建立新的柑橘中类柠檬苦素化合物检测方法,得到3种物质准确的质谱信息和含量.分析不同品种柑橘种子之间柠檬苦素、诺米林和奥巴叩酮的含量差异,为柑橘脱苦以及品种筛选培育提供依据.柑橘种子类柠檬苦素化合物的开发,不仅可以促进柑橘种植业及加工业的发展,同时可带动相关副产品加工业的发展,为柑橘的综合开发利用开辟了新的途径.
HTML
-
本研究所采用的柑橘果实于2014年11月采自中国农业科学院柑橘研究所国家种质资源圃.在果实成熟期,随机选择树势相对一致的3棵树,在树冠外围的中上部位随机摘取大小相对一致、成熟度相近、无病虫害的果实20个. 7种材料分别是:椪柑(Citrus reticulata Blanco. cv. ‘Ponkan’)、红橘(Citrus reticulata Blanco. cv. ‘Hongjv’)、常山胡柚(Citrus paradisi Macf. cv. ‘Changshanhuyou’)、梁平柚(Citrus grandis (L.) Osbeck. cv. ‘Liangpingyu’)、小香橼(Citrus medica L)、锦橙(Citrus sinensis (L.) Osbeck. cv. ‘Jincheng’)和宜昌橙(Citrus ichangensis Swingle).
-
标准品诺米林(纯度98.0)、奥巴叩酮(纯度98.0)和柠檬苦素(纯度98.0)购自成都克洛玛生物科技有限公司(中国,成都);色谱纯乙腈购自Sigma-Aldrich (St. Louis,MO,USA);甲酸购自Waters公司(Milford,MA,USA);超纯水由Milli-Q系统(Millipore,Bedford,MA,USA)制备;石油醚、二氯甲烷为分析纯试剂,购自四川成都科龙化工试剂厂(中国,成都);过滤膜(PTFE,0.22μm)购自ANPEL Inc(中国,上海).
-
电热恒温鼓风干燥箱DHG-9240A(上海齐欣科学仪器有限公司);小型粉碎机ZN-04A(北京兴时利和科技发展有限公司);电子天平JT3101N(感量0.1 mg,Max 3100g,上海精天电子仪器有限公司);电子天平Sartorius BSA 224S(感量0.1 mg,Max 220g,赛多利斯科学仪器(北京)有限公司);超声清洗器KQ5200DE(昆山市超声仪器有限公司);漩涡混合器DG-800(北京鼎国昌盛生物技术有限责任公司);台式低速大容量离心机TDL-5A(上海菲恰尔分析仪器有限公司);超纯水系统Milli-Q Advantage A10(美国Millipore公司);ACQUITY超高效液相色谱(UPLC)分析系统(美国Waters公司);Xevo G2-S Q-Tof高分辨四级杆飞行时间质谱仪(配有lockspray接口(美国Waters公司)、电喷雾离子源(ESI源),美国Waters公司);ACQUITY UPLC BEH C18色谱柱(2.1×100 mm,1.7 μm)(美国Waters公司).
-
柑橘果实采摘后用干净的抹布擦去外部灰尘,种子被分离,置于40 ℃鼓风干燥箱中干燥,种子烘干72 h,之后用小型粉碎机粉碎并过60目筛.干粉储存于-80 ℃冰箱中备用.
类柠檬苦素化合物的提取在原有方法上加以改进[25-26].平行称取冷冻干燥样品3份,每份样品约2 g(精确到0.0001)于100 mL锥形瓶中,加入20 mL石油醚,室温(25 ℃)超声提取60 min,5 000 r/min离心5min,合并固相,加入60mL二氯甲烷,锡箔纸封口,室温(25 ℃)超声提取30 min,抽滤,合并有机相于蒸馏瓶中,36 ℃下减压旋转蒸发至干,用1 mL乙腈溶解,转入1.5 mL离心管中,5 000 r/min离心10 min,上清液用乙腈定容至1.5 mL.测定样品时,将提取液过0.22 μm亲水性PTFE膜到2 mL进样小瓶,待测.
-
分别将标准品溶解于乙腈中并定容至10 g/L,作为标准品储备液.精密吸取标准品储备液,用乙腈分别定容至不同质量浓度:诺米林(0.050,0.25,0.50,1.0,2.0,2.5 g/L),奥巴叩酮(0.010,0.050,0.10,0.20,0.40,0.50 g/L),柠檬苦素(0.10,0.50,1.0,2.0,4.0,5.0 g/L).分析前,所有标准品储存于4 ℃冰箱中备用.
-
PDA检测器波长扫描范围为200~400 nm,根据文献报道[27]选择210 nm作为检测波长. UPLC-Q-TOF-MS系统使用ESI离子源,在正离子模式下全扫描采集数据.采用电喷雾电离离子源(ESI),毛细管电压0.45 kV,锥孔电压40 V,离子源温度120 ℃,除溶剂温度400 ℃,雾化气体为氮气(N2),碰撞气体为氩气(Ar),锥孔气体为流量50 L/h,脱溶剂气体流量800 L/h,低能量为6 V,梯度高能量为20~40 V;MS扫描范围m/z 100~1 000,扫描时间为0.2 s;质谱质量数校正溶液亮氨酸脑啡肽(m/z为556.2766,质量浓度为200 μg/L,流速为10 μL/min).
-
精密吸取3种类柠檬苦素的各质量浓度标准品溶液,分别进样.以峰面积为纵坐标(Y),质量浓度(X)为横坐标,绘制标准曲线,计算标准曲线的回归方程及相关系数.将标准品溶液逐步稀释,并以信噪比(S/N)为3时所对应的化合物质量浓度为检出限(LOD),以信噪比(S/N)为10时所对应的化合物质量浓度为定量限(LOQ).
-
精密度实验是在UPLC的基础上,评价所建立的分析方法的精密度.精密吸取3种类柠檬苦素标准品溶液,按已确定的分析方法进样.短时间内连续进样6次,测定类柠檬苦素标准品的峰面积,计算日内标准偏差,之后分别进样3次计算日间标准偏差.
-
平行称取冷冻干燥样品6份,分别加入一定量柠檬苦素、诺米林和奥巴叩酮标准品溶液,运用所建立的方法,对加标样品进行处理和分析.计算各加标样品的加标回收率及相应的相对标准偏差(RSD)值.
1.1. 实验材料
1.2. 实验试剂
1.3. 仪器设备
1.4. 实验方法
1.4.1. 样品制备
1.4.2. 标准品溶液配制
1.4.3. UPLC-Q-TOF-MS系统的条件
1.4.4. 线性关系考察及检出限、定量限测定
1.4.5. 精密度实验
1.4.6. 加标回收率
-
为找到最佳条件分析柑橘种子中类柠檬苦素,本实验优化了不同的UPLC条件,包括流动相与进样量、色谱柱温(30 ℃、35 ℃和40 ℃)和流动相流速(0.3 mL/min,0.4 mL/min和0.5 mL/min)等,最终优化所得最佳的分析条件是:流动相为0.01%甲酸+H2O(A),乙腈(B);洗脱程序为0~4.0 min,35%~55% B;4.0~5.0 min,55%~35% B;流速为0.4 mL/min,进样量为1.0 μL,色谱柱温度为40 ℃.
-
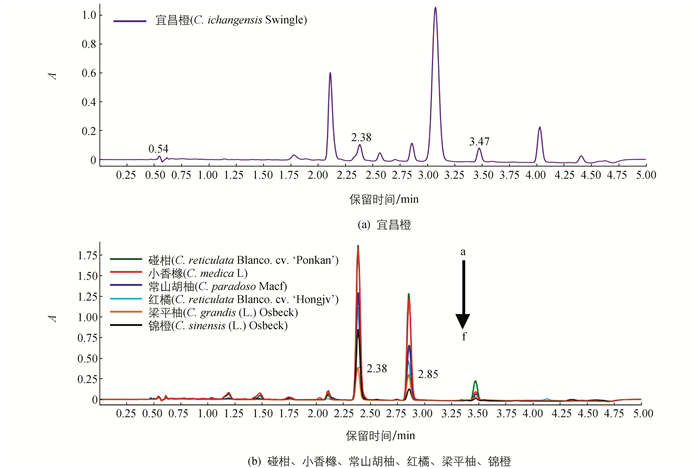
通过UPLC-Q-TOF-MS系统,7种柑橘种子中类柠檬苦素的分布得到确定.本实验中,各样品色谱峰分离情况良好.所有样品中均出现柠檬苦素、诺米林和奥巴叩酮的相应色谱峰(图 1).通过与标准品保留时间和MSE质谱数据(表 1和图 2)比较,柑橘种子中3种类柠檬苦素得以确定.柠檬苦素、诺米林和奥巴叩酮分别在2.38,2.85和3.47 min按洗脱顺序出峰.准确的分子质量在正离子模式下,由分子离子峰[M+H]+和加Na峰[M+Na]+确定,化合物分子结构由准分子离子和断裂产生的碎片离子确定.
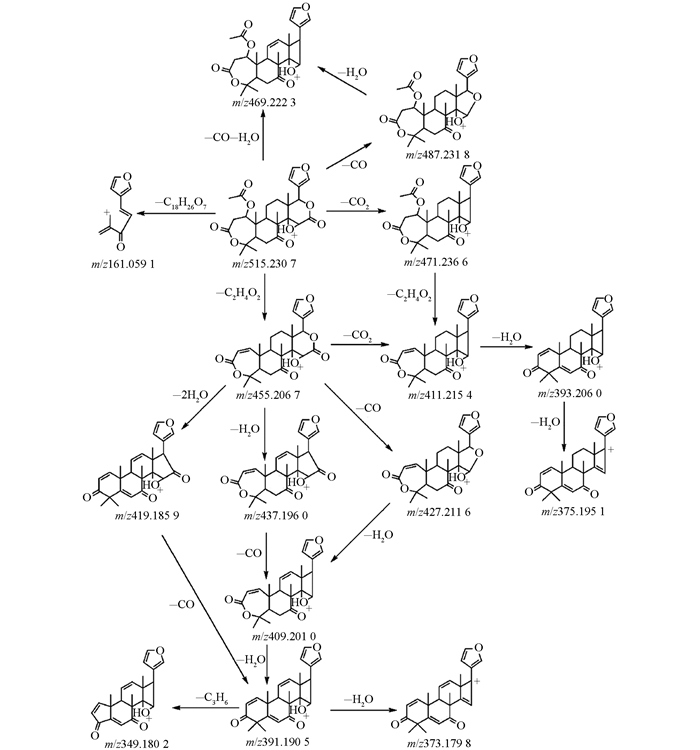
为研究3种类柠檬苦素碎片离子裂解规律,在正离子ESI模式下进行MSE扫描(图 2).在高能状态下所有碎片离子均能出现(表 1).柠檬苦素在正离子模式下进行MSE扫描,出现双分子离子峰[2M+1]+为m/z 941.395 6.正离子模式下特征碎片离子m/z 427.209 9,由准分子离子[M+H]+丢失CO2(44Da)得到.准分子离子丢失H2O(18Da)和CO(28Da)得到碎片离子m/z 425.196 0.同样,奥巴叩酮在正离子模式下,准分子离子[M+H]+丢失CO2(44Da)得到碎片离子m/z 411.216 1.准分子离子丢失H2O(18Da)和CO(28Da)得到碎片离子m/z 409.201 4.实验结果中,柠檬苦素和奥巴叩酮的质谱信息与裂解规律与之前报道相吻合[23, 28-29].因诺米林详细的质谱裂解途径尚未被报道,本实验基于正离子模式下高能状态碎片离子,对诺米林可能的质谱裂解途径加以讨论.
在正离子模式下诺米林出现较强碎片离子峰m/z 487.232 9,由准分子离子[M+H]+m/z 515.228 0丢失CO(28Da)形成.碎片离子[M+H-CO]+进一步失去H2O(18Da)形成m/z为469.222 3的碎片离子.准分子离子失去CH3COOH(60Da)形成同奥巴叩酮分子离子质荷比455.206 7相一致的碎片离子.诺米林在高能碰撞轰击下,主要丢失H2O、CO、CO2和CH3COOH等中性碎片.由于结构相近,3种类柠檬苦素的质谱裂解途径表现出极大的相似性.此外,3种类柠檬苦素都出现碎片离子峰m/z 161.06,并在高能状态下表现出较高强度,是呋喃环连接着内酯环断裂后留下的残基. MS/MS结果显示,CH3COOH(60Da)的丢失可能是因为发生了McLafferty重排.化学键的断裂失去H2O(18Da),CO(28Da)和CO2(44Da),主要发生在内酯环上.在UPLC-Q-TOF-MS系统中,正离子ESI模式下,诺米林的质谱裂解途径见图 3.
-
利用UPLC-PDA检测器建立了一种定量测定柑橘种子中柠檬苦素、诺米林和奥巴叩酮的方法.方法学考察结果见表 2和表 3.日内标准偏差与日间标准偏差的RSD分别低于2.2%,0.84%.诺米林、奥巴叩酮和柠檬苦素的加标回收率分别为95.71%,104.9%和107.7%.对应的RSD分别为0.56%,0.62%和0.95%.柠檬苦素、诺米林和奥巴叩酮的检出限(LOD)分别为3.0 mg/mL,3.0 mg/mL和0.5 mg/mL,而相应的定量限(LOQ)分别为5.0 mg/mL,8.0 mg/mL和1.0 mg/mL. 3种类柠檬苦素回归方程的相关系数(R2)均优于0.998 6,说明3种类柠檬苦素化合物线性关系良好.结果表明该分析方法准确可靠.
-
研究结果显示3种类柠檬苦素分布在7种柑橘种子中.结合标准品的标准曲线(表 3)对柑橘种子中类柠檬苦素的质量比进行测定(表 4),在7种柑橘种子中,柠檬苦素质量比最高,其次是诺米林,奥巴叩酮质量比最低.柠檬苦素质量比变化范围为165.57 μg/g (YCC)~257 8.8 μg/g (PK).诺米林质量比变化范围为164.97 μg/g (YCC)~1 691.8 μg/g (PK).奥巴叩酮质量比变化范围为10.752 μg/g (JC)~108.09 μg/g (PK).在7种柑橘品种中,椪柑种子中柠檬苦素、诺米林、奥巴叩酮质量比均为最高.而锦橙种子中奥巴叩酮质量比最低,宜昌橙种子中柠檬苦素和诺米林质量比最低.
宜昌橙中类柠檬苦素化合物的质量比和分布与其他6种柑橘品种相比,表现出其差异具有统计学意义. 6种柑橘种子中类柠檬苦素化合物的UPLC图谱有着一定的相似性(图 1).事实上,种子中类柠檬苦素化合物至少通过4种独立的生物合成途径,如柠檬苦素途径、卡拉敏途径、宜昌根辛途径和7-醋酸酯类柠檬苦素途径等[30].柠檬苦素途径存在于所有柑橘品种中.而宜昌根辛途径只存在于Citrus ichangensis种的植物品种及其杂交种的植物中[31].拥有该生物合成途径的植物组织中,去乙酰基诺米林和去乙酰基诺米林酸含量较高[4].作为一类分类学标记物,类柠檬苦素化合物广泛地存在于柑橘及其相近品种中,并表现出品种自身独有的分布特点[26].类柠檬苦素化合物的含量和分布的差异,揭示了植物本身遗传机制的不同.因为遗传的稳定性,分析这类化合物,可以推测出柑橘杂交种的亲本.而且分析柑橘以外的柑桔属植物的类柠檬苦素化合物,对筛选优良柑橘及其有杂交可能的植物和培育新品种具有重大意义.类柠檬苦素化合物的分析也可以为新品种的分类命名提供重要依据,并且能够对现有的分类方法进行评价.本研究中,7种柑橘种子的UPLC图谱显示宜昌橙与其他6种柑橘存在明显的差异,再次证明了类柠檬苦素化合物是一类很好的分类学标记物.
2.1. UPLC分析方法的建立
2.2. 类柠檬苦素化合物的定性分析
2.3. 方法学考察
2.4. 类柠檬苦素化合物定量分析
-
本研究基于UPLC-Q-TOF-MS优化出一种新的柑橘中类柠檬苦素化合物的定性定量检测方法,3种类柠檬苦素化合物在5 min之内得到有效分离,测定了7个不同品种柑橘种子中3种类柠檬苦素化合物的质量比,为商业检测标准提供了依据;得出3种物质ESI-Q-TOF-MS碎片离子规律,通过与标准品比较,7种柑橘种子中3种类柠檬苦素化合物得到鉴别;首次研究诺米林ESI-Q-TOF-MS质谱裂解途径;通过比较类柠檬苦素化合物的含量及分布,再次证明类柠檬苦素化合物可作为柑橘属植物分类学标记物,为柑橘品种选育提供依据.







 DownLoad:
DownLoad: