-
山葡萄(Vitis amurensis)为葡萄科葡萄属,耐旱、抗低温,是培育优良抗病菌、耐低温新品种葡萄稀有的种质资源[1-3],栽培范围最广泛的果树种类之一,在当今果树生产中具有重要地位,具有很高的经济价值.山葡萄果皮颜色能够影响果实品质,果皮的颜色主要受花色素苷组成的影响.
花色素苷(Anthocyanin)为类黄酮化合物,具有水溶性,是影响植物花、果实以及叶片颜色变化的重要成分.通过研究表明,果皮花色素苷质量浓度的多少决定果皮颜色的深浅,说明果皮色泽的差别与花色素苷的质量浓度相关.花色素苷功能很多,主要是使植物器官产生不同颜色,另外花色素苷对于种子传播、授粉、抵抗病原物侵染、防紫外线损伤等诸多方面都表现出明显作用[4-5].山葡萄因其含有很多可以作为天然色素的花色素苷,可以清除自由基、抗氧化、抗突变和抗疲劳而备受关注.因其栽培管理简单,产量较高,浆果易加工,酿酒工艺较简单,所以有非常好的市场前景.葡萄酒的品质也受花色素苷的影响,在酿造葡萄酒的过程中,多种花色素苷与其他物质相互作用,产生新的花色素苷,且在以后酿造过程中继续产生反应,生成许多复杂的花色素苷衍生物[6].因此对山葡萄花色素苷展开研究十分重要.花色素苷分布在植物的各个组织中,使植物呈现出色彩斑斓的颜色[7-8].但由于花色素苷稳定性影响因子多、降解机制复杂,结构不稳定,针对花色素苷的降解机制及提升花色素苷的稳定性进行研究极其重要.
山葡萄花色素苷的生物合成是一个复杂的过程,花色素苷合成分为两个途径:第一种途径是苯丙烷类代谢途径,第二种途径是类黄酮途径,其中需要很多酶参与,如肉桂酸-4-羟化酶(C4H)是苯丙烷类代谢途径的关键酶,也是植物中分布最广的主要CYP450之一,在花色素苷的形成过程中具有关键作用.与其他CYP450相比,C4H的一个明显特点是在植株的各个组织中均具有很高活性. C4H是第一个被鉴定的植物CYP450单加氧酶,也是第一个被克隆和确定功能的植物CYP450,迄今许多植物的C4H基因已被分离[9].本次实验用山葡萄作为基本材料,采用同源克隆的方法,克隆C4H基因的cDNA片段,得到全长序列.并应用农杆菌介导拟南芥遗传转化方法将农杆菌菌液与植株的花序接触,得到拟南芥转化体,经检测初步验证了目的基因C4H的功能和活性.利用生物学技术来研究C4H基因在山葡萄着色过程中的作用,更准确地揭示山葡萄果皮着色的分子机理.
HTML
-
山葡萄品种“双丰” (Vitis amurensis cv. Shuang Feng)采自延边大学农学院山葡萄种质资源圃.在果实成熟期采摘,采后立即置于液氮中冷冻,-80 ℃冰箱保存,取果皮作为供试材料.拟南芥(Arabidopsis thaliana)由延边大学提供.
实验所用大肠杆菌(Escherichia coli) DH5-α,pMD18-T载体,2xTaq Plus PCR MasterMix产品购于天根生物有限公司;反转录酶SuperScriptⅡ购于Invitrogen公司;胶回收试剂盒购于Promega公司;实时荧光定量PCR试剂盒购于天根公司;限制性内切酶购于Promega公司,T4连接酶、卡那霉素、羧苄青霉素、庆大霉素和Silwet L-77购于上海生工生物公司;其他试剂为国产分析纯.根癌农杆菌(Agrobacterium tumefaciens)为GV3101,表达载体为pCAMBIA1301(延边大学农学院提供).
-
山葡萄果皮总RNA提取采用改良CTAB法[10].提取后用1%琼脂胶检测RNA完整性,检测RNA浓度、纯度.合格的RNA采用SuperScriptⅡ反转录酶合成cDNA,具体操作参照说明书.
-
在NCBI网站中,下载GenBank中与山葡萄近缘植物的C4H基因的基因序列,使用多序列比对方法找到保守区域,利用Primer6.0设计各特异引物.引物序列如下:
C4H-s:5′- ATCCACCGCCACAACCAT-3′;C4H-a:5′- AGTTTCCCATCTTCATCTTT-3′,在上海生工生物公司合成.利用C4H基因引物进行PCR扩增来获得目的基因片段. PCR反应采用25 μL体系,其中含ddH2O,10.5 μL;2xTaq Plus PCR MasterMix,12.5 μL;引物各0.5 μL;模板cDNA,1 μL.扩增程序为94 ℃预变性3 min,94 ℃ 30 s,58 ℃ 1 min,72 ℃ 2 min,32个循环,最后72 ℃延伸5 min.对目的基因片段进行胶回收(参照试剂盒说明书操作),连接pMD18-T载体,转化大肠杆菌DH5-α感受态细胞,蓝白斑筛选,挑取阳性克隆测序.
-
测序后得到一个全长序列,并利用生物信息学软件进行序列分析,用到的软件如表 1.
-
遵循实时荧光定量引物设计要求设计qRT-PCR引物,并保证引物特异性,引物序列如下:qC4H-F:5′-TGGCTTGCTAACGACTC-3′;qC4H-R:5′- CAATGGTGGAATGCTTC -3′,由上海生工生物公司合成.分别以8个时期的山葡萄果皮RNA反转录的cDNA为模版,以ACTIN基因为内参基因进行qRT-PCR分析.根据TIANGEN实时荧光定量说明书,反应体系:2×SuperReal Premix Plus 10 μL,10 μmol/L的正反向引物各0.6 μL,cDNA模版1 μL,50×POX Reference Dye 0.4 μL,RNase-free ddH2O补至总体积为20 μL;反应程序:95 ℃预变性15 min,95 ℃变性10 s,56 ℃退火32 s,60 ℃延伸32 s,循环40次.每个样品设置3个重复.数据应用MxPro软件处理.
-
根据已克隆出的山葡萄C4H的ORF序列,设计含有酶切位点的特异扩增引物:F:5′-CGGAATTCCTAACGGCAGTGCAGCTTGC-3′(含EcoRI酶切位点);R:5′- TCCCGGGTCAAGCTTCTATTGGCCTTGCCACAAT -3′(含SmaI酶切位点),以山葡萄C4H全长cDNA为模版,利用设计的引物PCR扩增山葡萄C4H序列,扩增程序为94 ℃预变性3 min,94 ℃变性30 s,59 ℃退火1 min,72 ℃延伸2 min,35个循环,最后72 ℃延伸5 min.将含有酶切位点的山葡萄C4H与pET-28a原核表达载体进行双酶切,回收目的基因片段和原核表达载体大片段,并按一定体系用T4-DNA连接酶16 ℃连接过夜,转化到感受态细胞,涂布于100 mg/L卡那霉素抗性固体LB培养基上,37 ℃恒温培养箱中培养12 h,挑取单克隆进行PCR和双酶切鉴定.构建重组表达载体pET28a-VAmC4H,经不同浓度的IPTG诱导8 h,用SDS-PAGE(15%)检测目的蛋白的表达情况,以未诱导的重组表达载体为对照.
-
在目的基因C4H 的cDNA两端加上EcoRI和SmaI酶切位点,引物分别为正向:5′-CGGAATTCATGGATCCCATACTCATAGAGAAAGCTCTGT-3′;反向:5′-TCCCGGGTCAAGCTTCTATTGGCCTTGCCACAAT-3′,用上述两种酶对目的基因C4H进行双酶切,将其与pCAMBIA1301双酶切产物用T4连接酶在16 ℃下过夜连接,进行表达载体构建;将连接产物转化至感受态中,进行阳性克隆筛选,提取表面载体pC C4H,应用冻融转化法转入到GV3101农杆菌中,以目的基因序列为引物(C4H-s:5′- ATCCACCGCCACAACCAT-3′;C4H-a:5′- AGTTTCCCATCTTCATCTTT-3′)进行PCR验证(目的基因序列为1,735 bp,GeneBank序列号MH045992),测序后证实GV3101农杆菌与目的基因pC C4H的表达载体构建成功,命名为GV3101/pC C4H.
-
将拟南芥种子春化处理后,进行播种.除去长出的第一个花序,促进更多的花序能够萌发,花序在此萌发时,进行转化;转化液为B5培养基的维生素,0.044 μmol/L的BA,1/2 MS培养基的无机盐,50 μL/L的Silwet L-77,5%(W/V)的蔗糖;取GV3101/pCC4H菌液300 mL,8 000 r/min离心10 min,去上清;加入500 mL转化液,重悬沉淀,浸染拟南芥的花序,浸泡约2 min,连续3次;约4周能够收获种子.
-
1/2 MS固体培养基中加入50 mg/L Kan,将经春化处理的转化的拟南芥种子用0.1%氯化汞消毒处理后,按每板300粒种子接种于1/2 MS固体培养基上(含50 mg/L Kan);适宜条件下,光照培养箱中培养,观察其生长状况;筛选具有抗性的生长正常的植株用于后续实验.
-
具体操作方法见参考文献[11].
-
拟南芥总RNA提取后用1%琼脂胶检测RNA的完整性,检测RNA的浓度、纯度.合格的RNA采用SuperScriptⅡ反转录酶合成cDNA,具体操作参照说明书.以拟南芥cDNA为模版,目的基因序列为引物,PCR反应采用25 μL体系,其中含ddH2O,10.5 μL;2xTaq Plus PCR MasterMix,12.5 μL;引物各0.5 μL;模板cDNA,1 μL.扩增程序为94 ℃预变性3min,94 ℃ 30 s,58 ℃ 1 min,72 ℃ 2 min,32个循环,最后72 ℃延伸5 min.
-
用直径为1 cm的打孔器随机取3块叶片,放入1.5mL的0.1%的盐酸甲醇溶液中于黑暗-20 ℃提取24 h,5 000 r/min离心5min,取上清于535nm处测定其吸光值[12],紫外可见分光光度计为Ultrospe 3000(Amersham Pharmacia Biotch),用公式A535=A535-0.25A657来校正花色素苷的质量浓度[13-14].
1.1. 材料
1.2. 方法
1.2.1. RNA提取及cDNA第一链的合成
1.2.2. 山葡萄C4H基因的克隆
1.2.3. 山葡萄C4H基因cDNA序列及其编码蛋白氨基酸的序列分析
1.2.4. 山葡萄果皮不同着色时期C4H的表达分析
1.2.5. 原核表达载体的构建和表达
1.2.6. 真核表达载体的构建与转化
1.3. 拟南芥的遗传转化及筛选
1.3.1. 拟南芥的遗传转化
1.3.2. 转化体的筛选
1.4. 拟南芥RNA的提取及PCR检测
1.4.1. 拟南芥基因组RNA的提取
1.4.2. cDNA的制备及PCR检测
1.5. 测定拟南芥中花色素苷的质量浓度
-
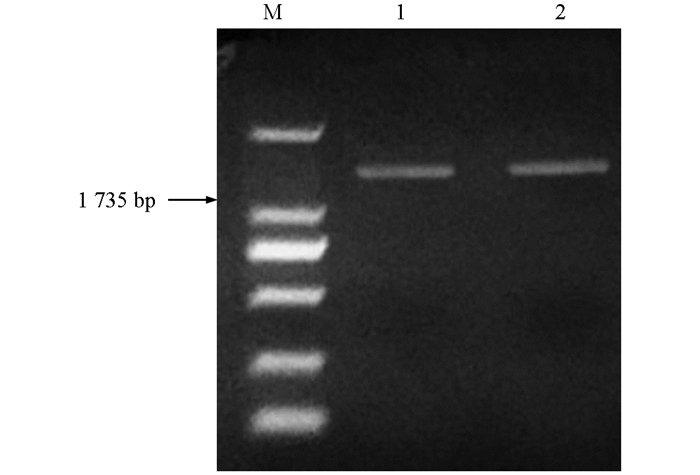
采用改良的CTAB法[10]提取成熟期葡萄果皮中的总RNA,将其反转录合成cDNA模板,用C4H基因引物C4H-s和C4H-a进行PCR扩增,获得了目的特异条带,约1 735 bp左右,与预期片段大小相近(图 1).产物经连接转化、筛选测序后获得保守序列片段,用NCBI/BLAST程序进行同源搜索,结果与欧亚种葡萄(XM_002266202)同源性达到99%,初步认定该序列为山葡萄C4H基因的核苷酸序列.
-
运用DNAstar软件对山葡萄C4H基因进行开放阅读框分析,结果表明,该基因开放阅读框1 518 bp.利用ProParam软件推测基因的开放阅读框编码505个氨基酸,分子质量为57.70 KDa,等电点值9.06.在构成山葡萄C4H蛋白的20种氨基酸里面亮氨酸(Leu)的量最多,为11.7%;半胱氨酸(Cys)的量最少,为0.8%.正电荷残基(Arg+Lys)个数是69,负电荷残基(Asp+Glu)个数是61,不稳定性系数是46.97,脂溶系数是100.16,说明山葡萄C4H蛋白为稳定蛋白质.将该基因命名为VAmC4H,GenBank登录号为MH045992.
-
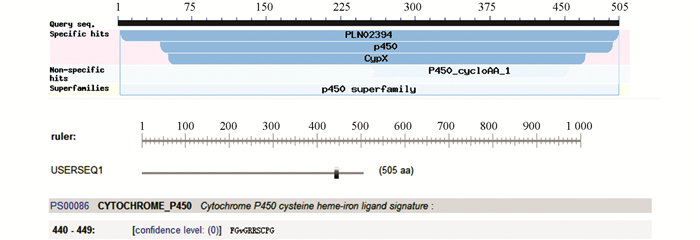
对VAmC4H蛋白保守区进行预测,具有P450超级家族结构域,属于C4H家族蛋白.其特征序列为FGVGRRSCPG,位于该蛋白的440-449氨基酸位置(图 2).
-
使用TargetP 1.1软件对VAmC4H蛋白序列进行细胞定位分析,分析结果显示蛋白在线粒体中可能性是0.012,在分泌途径中存在的可能性是0.983.可靠级别为1,见表 2.
-
使用NCBI的工具BlastP对获得的山葡萄C4H基因的氨基酸序列做同源性比对.比对结果表明,山葡萄C4H基因同欧亚种葡萄(XM_002266202)、爬山虎(ABA59555)等C4H基因的同源性相近,相似性分别为99%,97%.运用多重序列比对软件,将山葡萄VAmC4H及其他植物C4H基因编码的氨基酸序列比对分析,结果如图 3.
-
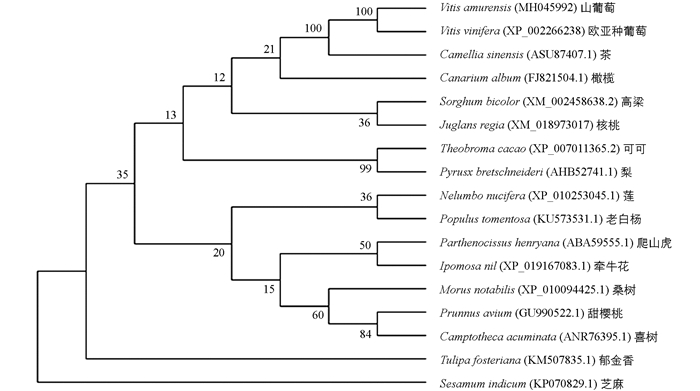
为了对VAmC4H和其他物种的C4H基因做深入的研究,在多重比对的基础上,使用分子生物学软件MEGA 5.0,对VAmC4H基因及其他物种C4H基因的氨基酸序列构建系统发生进化树.分析结果如图 4,VAmC4H基因同欧亚种葡萄同源性最近,同可可、莲等植物的同源性较低.
-
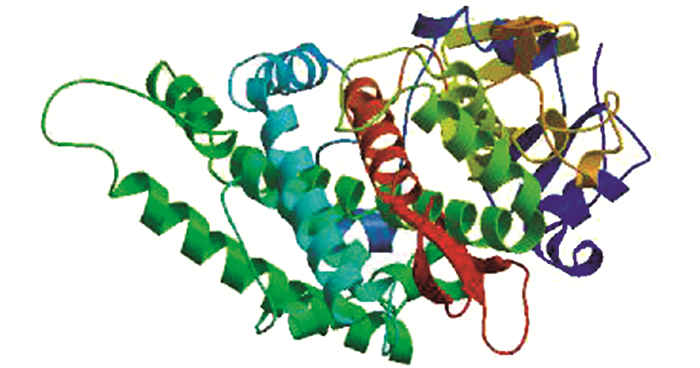
运用网上资源HNN推测VAmC4H的二级结构(图 5),结果表明该蛋白的二级结构是由40.59%的无规卷曲,48.32%的α-螺旋和11.09%的β-折叠构成.利用SWISS-MODEL预测VAmC4H的三级结构,见图 6.
-
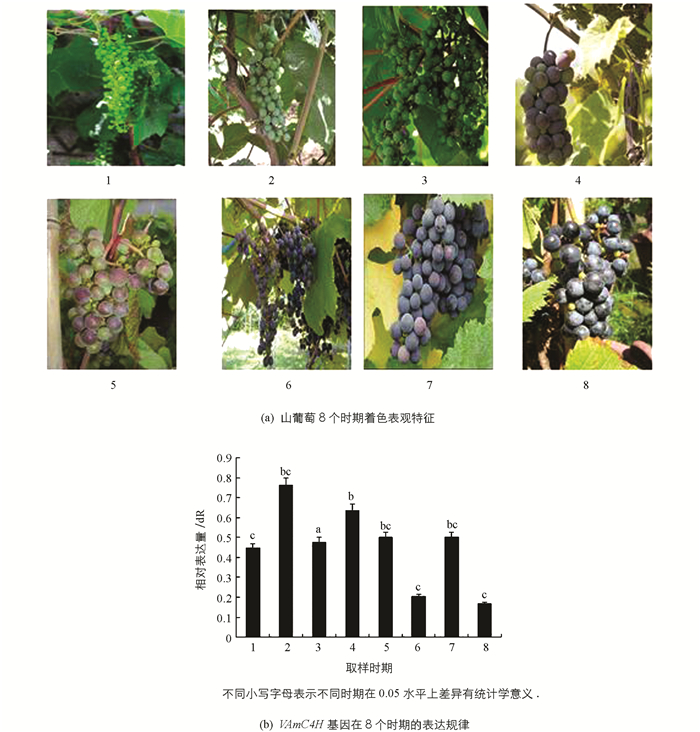
利用实时荧光定量PCR检测VAmC4H基因在山葡萄果皮8个不同转色时期的表达量,结果表明VAmC4H在山葡萄果皮转色各个时期均存在表达,C4H基因表达量在整个生长过程呈不规则变化,见图 7.
-
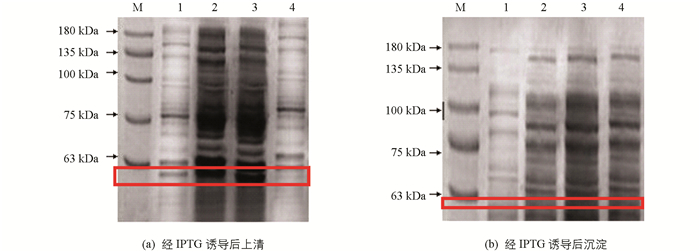
将重组质粒pET28a-VAmC4H转化至感受态细胞BL21后,加入IPTG诱导菌体表达.在28 ℃培养条件下对IPTG浓度进行筛选,分别为0.8 mmol/L,1.0 mmol/L,1.2 mmol/L,并进行SDS-PAGE电泳检测(图 8).目的蛋白在上清中且IPTG诱导浓度为0.8 mmol/L,1.0 mmol/L(图 8a),约57.70 kDa处有1条与预测重组VAmC4H蛋白相对分子质量大小一致的较高浓度的诱导表达带,沉淀与对照组均未出现目的蛋白条带(图 8b).表明VAmC4H基因在大肠杆菌中已成功表达.
-
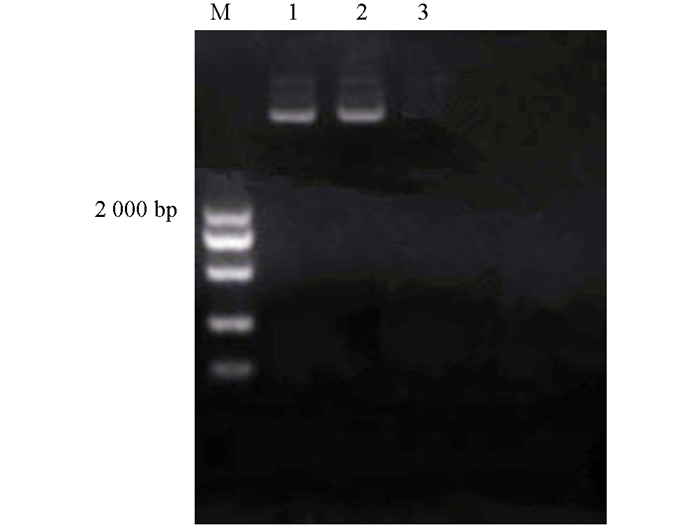
利用冻融热击法将pC C4H质粒转化至农杆菌GV3101中,涂布于含有Kan的平板中进行筛选,单菌落进行阳性PCR检测(图 9),选阳性菌落接种转拟南芥花序.收获成熟拟南芥种子,春化后,在1/2 MS培养基(含50 mg/L Kan)上进行筛选,得到3株阳性拟南芥幼苗.
-
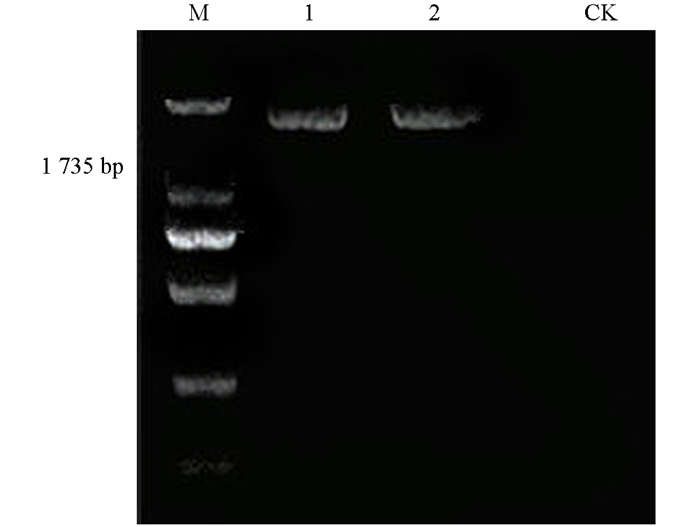
对具有抗性的转基因植株PCR检测,提取叶片中RNA进行反转录,以其cDNA为模版、目的基因序列为引物,PCR检测结果,2株抗性植株均为阳性,扩增的目标条带约为1 735 bp(图 10).
-
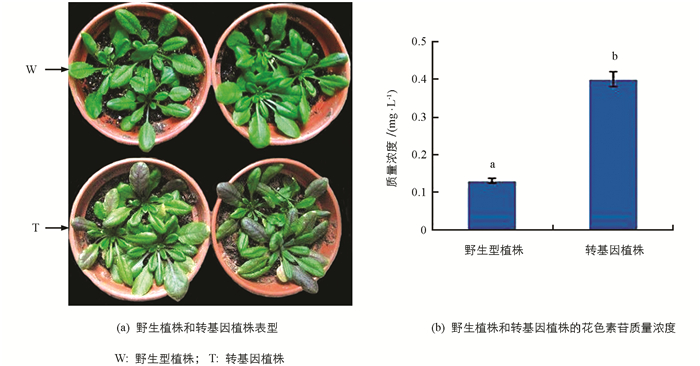
从2株阳性植株的表观颜色来看,茎杆和叶片的颜色均发生了变化,茎杆和叶片转变为浅紫红色.对转化型和野生型拟南芥植株进行了花色素苷质量浓度的粗测定,前者质量浓度比对照野生型约高3倍(图 11).
2.1. RT-PCR合成山葡萄C4H基因cDNA全长序列
2.2. cDNA全长序列生物信息学分析
2.2.1. 基因编码蛋白质理化性状分析
2.2.2. VAmC4H蛋白保守区预测
2.2.3. VAmC4H蛋白的细胞定位
2.2.4. VAmC4H编码蛋白质的同源性
2.2.5. VAmC4H编码蛋白质的进化分析
2.2.6. VAmC4H蛋白二级结构和三级结构预测
2.3. VAmC4H基因在山葡萄果皮不同转色时期的表达分析
2.4. VAmC4H编码产物的原核表达
2.5. 转化农杆菌
2.6. 转基因植株PCR检测
2.7. 转基因植株表型和花色素苷质量浓度
-
苯丙烷类代谢途径是植物体次生代谢中的一个通用途径,在植物生长发育中具有重要作用[15].通常认为此途径参与一系列生物反应形成花色素苷并由几种酶共同参与完成催化[16].肉桂酸-4-羟基化酶(C4H),又称反式肉桂酸-4-单氧化酶,催化肉桂酸羟化作用产生4-香豆酸盐,是苯丙烷途径中继L-苯丙氨酸解氨酶(PAL)之后的第二个关键酶.很多植物的C4H大多以基因家族形式存在.迄今,已有多种植物的C4H基因被分离出来[17-19]. C4H基因拷贝数在不同植物中呈现不同,苜蓿有2个C4H基因,豌豆(Pisumsal sativum)只有1个拷贝,长春花(Catharanthus roseus)和绿豆的C4H是由多个拷贝组成的1个小的基因家族,这些基因编码的氨基酸序列同源性普遍较高,约85%.在山葡萄中C4H基因的存在及功能尚未被报道,该基因拷贝数仍需进一步验证.本实验借鉴前人方法与经验,最初在山葡萄里克隆得到其C4H基因cDNA全长序列,且命为VAmC4H.用生物信息学软件进行多序列比对分析,预测其蛋白的二级结构发现,它由无规卷曲,α-螺旋,β-折叠构成,此结果与前人得出的葡萄、爬山虎等C4H蛋白二级结构结论相同,表明VAmC4H和其他植物已分离C4H基因有极高同源性,尤其是欧亚种葡萄,并具有相同蛋白质保守活性位点.该基因参与催化植物的次生代谢,因此研究其生物学活性能够有效进行品种培育和植物遗传改良.
VAmC4H在山葡萄果皮转色各个时期时均存在表达量,因此推测该基因可能在植物的生长发育过程中,各时期都参与苯丙烷类代谢途径合成花色素苷.本研究选取C4H基因家族共同保守区域进行表达检测,可证明C4H基因家族在山葡萄果皮各转色时期具有普遍规律. BL21(DE3)菌株是实验室应用最为广泛的表达菌株[20],很多外源基因都能够在该菌株中大量表达,VAmC4H蛋白在上清中有较高浓度的诱导表达带,表明VAmC4H基因在大肠杆菌中已成功表达.在某些植物中,该酶和PAL样常由于植物组织的损伤、光照或培养细胞的老化等因素而影响诱导活性[9, 21].而在拟南芥中花色素苷质量浓度虽然较低,但依然有少量合成,证明该基因影响花色素苷生物合成途径,但积累的量不多;转基因拟南芥中的C4H基因过量表达,能促使花色素苷的合成并快速积累,进一步提高花色素苷合成的稳定性.有学者认为C4H是一个调控酶,在植物幼苗中以及正在经历主动木质化的组织中表达水平较高.它的催化活性通常在受损的组织中增加,通过乙烯处理可以增强该酶的活性[22].在光照和病菌感染后其活性也会增强[9, 23].在生物合成途径中,C4H基因起到了与各个分支反应相联系的纽带作用. C4H参与次生代谢物质合成,表现出时间和部位上的差异,在植物生长代谢各个时期这个基因表达旺盛[24]. C4H是苯丙烷类代谢途径的调控位点,其活性的高低也是调节苯丙烷类代谢途径碳通量的最重要的因素,因此,C4H的结构-功能关系和酶活机理是近年来备受重视的研究热点[25].随着分子生物学的快速发展,已初步揭示生物合成的大体轮廓,并逐步应用到不同植物资源的研究中.







 DownLoad:
DownLoad: