-
茄子(Solanum melongena L.),又名落苏、茄瓜,是一种受广大消费者喜欢的1年生草本植物,具有较高的营养价值,为世界主要的大宗蔬菜之一.为满足人们更高的需求,对茄子优质品种的选育成为研究热点之一.为加快育种进程,国内外对茄子离体再生进行了大量研究. 1969年,Ono等通过合子胚诱导产生愈伤组织,获得了再生植株,在此之后,茄子各器官几乎都有组织培养和植株再生的研究[1]. Sharma等通过对同植株的不同组织部位的诱导率进行对比研究发现,茄子的子叶和真叶部位更倾向于被诱导形成体细胞胚,与之相反,下胚轴则更易于诱导不定芽的形成[2]. Guri等则首先通过农杆菌介导的方式获得了转基因茄子[3],之后陆续有多位科学家通过转基因技术获得了抗虫、抗病的转基因茄子[4-8].国内的科学家也利用不同品种茄子的子叶和下胚轴进行了离体培养的研究[9-11].经多年研究,虽然茄子的遗传转化技术得以提高和改善,但仍存在转化效率较低的问题,且普遍低于同科的番茄和烟草,导致后续研究需要投入较多的财力、物力和人力,造成资源浪费,在研究成果的市场化应用上形成技术壁垒.本研究在原有遗传转化体系基础上,研究改进了以栽培种三月茄为材料的茄子转基因遗传转化体系,为后期茄子遗传性状改良和种质资源开发奠定基础.
HTML
-
以普通三月茄为试验材料,转化选用的CRISPR敲除载体pKSE401和农杆菌菌株LBA4404均由西南大学重庆市蔬菜重点实验室保藏.
-
通过对比已有研究和实验室前期摸索,笔者选用农杆菌LBA4404为转化菌株.利用冻融法转化农杆菌,经PCR鉴定获得pKSE401的农杆菌单克隆.
-
筛选出色泽亮丽、表面光滑、颗粒饱满的茄子种子,先使用20% NaClO浸泡1 min,充分打湿即可,后用双蒸水将其冲洗3~5遍,再将其置于55~60 ℃的温水中进行温汤浸种,20 min后取出,在超净台上依次用75%酒精浸泡1 min,无菌水冲洗3~5遍,浓度为30%的NaClO处理15 min后倒掉,再用新的30% NaClO浸泡20 min,无菌水洗8~10遍,直至水质透明清亮、无异色和杂质为止.完成消毒的种子密封于有少量无菌水的容器瓶中,放置于4 ℃冰箱中保存1~2 d. 1~2 d后将其转置于28 ℃恒温摇床中,在遮光条件下进行催芽,待种子露白后便可将其播种于MS培养基上,置于温度26 ℃、光照时长为16 h、光强约为2 000~3 000 lx的组培室中培养.
-
以普通三月茄无菌苗为试材,待子叶展平时,切取子叶和下胚轴接种到不同激素配比的培养基上进行培养.挑取平板上培养的农杆菌pKSE401阳性单菌落接种于YEB液体培养基中,28 ℃,220 r/min培养2 d.之后取500 μL上述培养液到50 mL YEB液体培养基中过夜培养至OD600 1.6~2.0.将经过2 d预培养的子叶和下胚轴在菌液中侵染10~15 min,期间不时摇晃使其保持充分接触,用无菌滤纸吸干水分后放回之前的预培养基中共培养2 d,之后转入不同抗生素配比和激素配比的诱导分化培养基中进行诱导培养.
-
以高温高压灭菌后的MS固体培养基为基本培养基,以实验室常用抗生素浓度(Carb 500 mg/L+Kan 100 mg/L)按照表 1的激素种类和配比制备成不同的诱导分化培养基.在子叶刚展平时进行处理,培养条件与无菌苗的制备环境条件相同,每一种处理设置3个重复,每一重复有3个培养皿,每个培养皿接种15片,培养8周,每4周更换一次培养基.连续观察并分别统计记录愈伤组织、不定芽和根的诱导分化情况,通过数据比较筛选出最佳外源激素诱导分化的种类与浓度配比组合.
出愈率是指已形成愈伤组织的外植体数量占接种的外植体总数之比例.出愈率可以反映出不同培养基诱导能力的差异,可作为观察指标,进一步筛选出最优培养基组合.
不定芽分化率是指在上一步骤中得到的愈伤组织,经过诱导分化产生不定芽的外植体个数所占接种的总外植体数的比例.不定芽的诱导率也可作为衡量该种培养基诱导茄子愈伤组织分化能力的重要指标.
-
待无菌苗子叶展平时,分别切取其子叶、下胚轴部位,子叶切取方法同激素筛选时方法一致,下胚轴取距离子叶基部0.5~1 cm处、长约1 cm的小段,分别置于表 1中各个处理的培养基中培养,下胚轴横放,不同外植体的数量、培养时间、培养条件的设置与1.2.4中相同,连续观察并统计记录子叶和下胚轴各自的分化情况.
-
组织培养过程中为确保试验材料不受污染,一般需在培养基中添加抗生素来抑制杂菌的生长,但不同种类和浓度的抗生素对杂菌抑制效果不同,甚至会影响外植体的分化.因此,在最佳激素配比下,以外植体的污染率和不定芽诱导率为指标对培养基中抗生素的浓度进行梯度筛选(图 2).本试验同样设置7个处理,每个处理3次重复,每次重复有3个培养皿,每个培养皿接种15片,培养4周,连续观察污染的外植体个数和不定芽诱导数,并进行统计记录.
染菌率是指在组织培养过程中,培养基中出现细菌、真菌等导致污染的外植体个数占接种的外植体总数的比例.染菌率可以较准确反映出不同的抗生素种类及浓度组合在抑菌效果上的差异性,可作为重要的衡量指标.
-
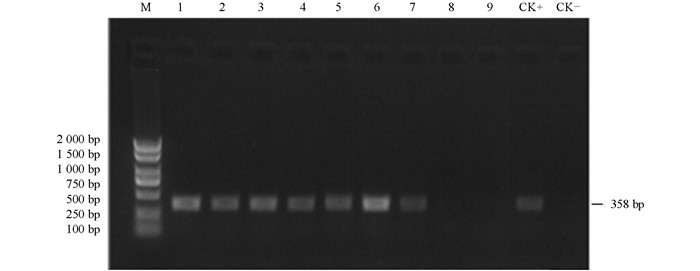
在获得转基因再生植株之后,提取植物总DNA通过PCR手段鉴定该植株是否为转基因阳性植株.检测使用Kan基因的特异性引物:
F:5’-AGGATCTCGTCGTGACCCAC-3’;
R:5’-GCACGAGGAAGCGGTCA-3’.
转化率是指已转化为转基因完整植株占外植体总数的比例.
1.1. 试验材料
1.2. 试验方法
1.2.1. 农杆菌的转化
1.2.2. 无菌苗的培养
1.2.3. 农杆菌介导的茄子遗传转化
1.2.4. 不同外源激素种类和浓度配比对诱导出芽
1.2.5. 不同外植体器官部位对分化的影响
1.2.6. 不同抗生素种类及其浓度配比的筛选
1.2.7. 农杆菌介导的茄子遗传转化率的统计方法
-
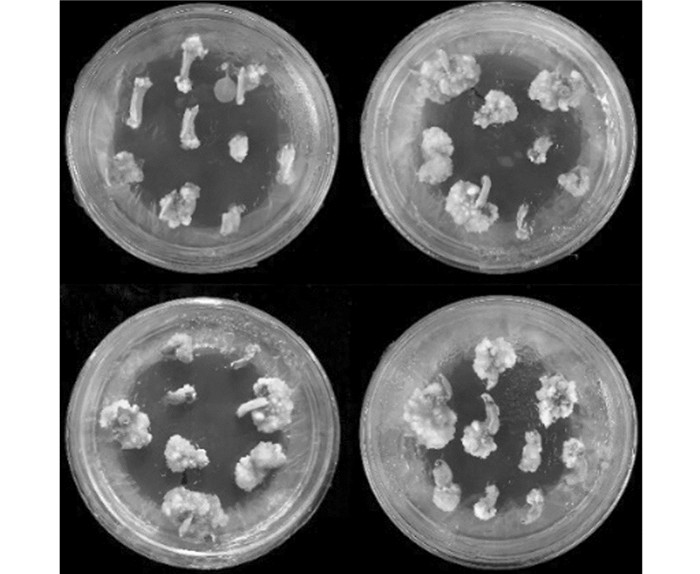
试验结果看出,在不同激素和抗生素配比下成功获得了数量不等的转基因茄子愈伤(图 1)和转基因再生植株(图 2).
从表 3可以看出,A~L的12个处理中,A~C组合诱导效果比较好,即当IAA浓度为0时,茄子子叶和下胚轴的出愈伤率均高于其余对照组,且当ZT浓度为1.0 mg/L时,子叶和下胚轴的出愈伤率及不定芽诱导率均达到全对照组中最佳状态;随着IAA和ZT浓度都增加,外植体的出愈伤率和不定芽诱导率均出现一定范围的下降.与此同时,根据数据发现,子叶的出愈伤率和不定芽诱导率均明显高于下胚轴,说明子叶更适于作为茄子外植体培养的供试材料,故筛选出外源激素最佳种类和浓度配比组合为MS+6-BA 2.0 mg/L+ZT 1.0 mg/L.
-
试验结果看出,绝大多数试验组污染率低于20%,所有组出愈率高于74%.其中Carb 250 mg/L+Kan 100 mg/L的组合污染率最低,相比之下能够达到最佳的抑菌效果;500 mg/L Carb+50 mg/L Kan的组合污染率最高,达到20.74%.此外,愈伤率从最低74.07%到最高82.96%不等,说明在一定范围内,适当降低Carb/Kan浓度的比值,可以提高出愈率(表 4).
-
本试验结果看出,子叶作为外植体时,获得的完整植株有207株,下胚轴作为外植体时,获得具有卡那抗性的完整植株92株,比例为2.25:1.据此比例,分别从中随机选取80株和35株作为样本提取DNA,进行PCR检测,检测到成功转化的植株分别为69株和27株(图 3).据此进行计算,转化率分别达到了11.02%和4.38%.
2.1. 不同激素种类及浓度对外植体子叶和下胚轴诱导分化的影响
2.2. 不同抗生素种类及浓度配比对茄子组培污染率和诱导分化的影响
2.3. 农杆菌介导的茄子遗传转化率
-
与番茄等蔬菜相比,我国茄子的基因工程研究一直相对落后,其中茄子转基因研究中的茄子芽分化率不高,转基因普遍转化率不高一直是制约其发展的重要因素.激素的配比在植物离体培养和不定芽分化中起决定性作用,王凤华等通过试验比较认为一定浓度的6-BA和NAA组合能诱导茄子不定芽的分化[11],余波澜等则用IAA与6-BA配合诱导茄子的分化[9].俞琼等的研究显示,子叶不定芽诱导的最适培养基为MS+KT 2.0 mg/L+NAA 0.2 mg/L,不定芽为MS+6-BA 2.5 mg/L+NAA 0.2 mg/L[12].张兴国等2001年通过试验最早建立了以6-BA 1 mg/L、ZT 2 mg/L、卡那霉素150 mg/L和羧苄青霉素300 mg/L为抗生素激素配比的三月茄转基因体系,但转化率仅为6.8%[13].近些年国内外也有利用转基因手段研究培育茄子新品种的报道,比如Wan等通过将拟南芥AtCBF3和AtCOR15A导入茄子基因组,获得了抗寒性更强的转基因茄子[14];Yarra等将小麦TaNHX2基因转入茄子提高了植物的耐盐性[15].但整体而言该类研究都相对较少,同时文中报道的转化效率都普遍较低.本试验通过对激素配比和抗生素种类和浓度的大量探索,选择出了最适合栽培种“三月茄”的激素和抗生素配比,将子叶和下胚轴的转化率分别提高到了11.02%和4.38%.
本试验结果显示,MS+6-BA 2.0 mg/L+ZT 1.0 mg/L+Carb 250 mg/L+Kan 100 mg/L更加适合三月茄子叶和下胚轴不定芽的诱导和分化.这一激素和抗生素配比与张明华等2014年对长茄进行遗传转化体系的研究结果基本吻合[16],但与其相反的是本试验发现三月茄中不同激素对子叶的诱导率均高于下胚轴,推测这可能是由于不同品种茄子间的差异导致,但具体原因还有待进一步研究.同时在大麦、棉花、玉米等作物上的研究表明,不同品种的材料由于基因型的差异,遗传转化体系也存在很大的差异[17],尤其是激素配比,因此还需要对更多品种进行遗传转化的研究来寻找规律以获得适应性更广的遗传转化体系.







 DownLoad:
DownLoad: