-
开放科学(资源服务)标志码(OSID):

-
氰戊菊酯(Fenvalerate,Fen)是一种Ⅱ型拟除虫菊酯类农药,因其具有高效、低毒、低残留等特点,而被广泛应用于农业生产和家庭生活中,但其对人类的生殖健康具有明显的危害性. 研究表明,氰戊菊酯是一种环境内分泌干扰物[1],具有明显的雄性生殖毒性,可通过影响睾酮合成相关酶和蛋白的表达而影响雄性体内睾酮的含量,以及影响支持细胞和间质细胞的功能来干扰下丘脑-垂体-性腺轴的调节作用[2-5],使雄性生精过程受损,导致精子数量减少及活力降低. 此外,研究表明,雄性体内的雌激素对雄性生殖功能也具有重要调节作用[6],氰戊菊酯在干扰睾酮分泌的同时,是否也干扰了雄性体内的雌激素分泌以及其受体的表达从而产生生殖毒性?为了验证我们的猜想,本研究拟通过分析雌激素及其受体ERβ在Fen染毒后的表达情况,探讨Fen等拟除虫菊酯类农药杀虫剂所致雄性生殖毒性的相关机制.
HTML
-
Fen原药(纯度98.80%),北京坛墨质检科技有限公司产品;HE染色试剂盒,北京索莱宝科技有限公司;雌二醇(E2) ELISA试剂盒,上海江莱生物科技有限公司;BCA蛋白浓度测定试剂盒,北京索莱宝科技有限公司;CYP19A1,Caspase 3,Caspase 9抗体,英国Abcam公司;ERβ,Bax,Bcl-2抗体,武汉三鹰生物技术有限公司;RNA逆转试剂盒和SYBR Premix Ex Taq,日本Takara公司;TUNEL细胞凋亡检测试剂盒(增强型绿光),武汉伊莱瑞特生物科技公司.
-
低温高速离心机,美国Thermo公司;分光光度计(ND2000C型),美国Thermo公司;酶标仪,美国Bio-Rad公司;显影仪,美国Syngene公司;PCR仪,美国Life Technogies公司;荧光显微镜,日本Olympus公司.
-
体质量220~250 g健康性成熟的雄性SD大鼠24只,购自重庆腾鑫生物技术有限公司,动物许可证号:SCXK(辽)2015-0001. 大鼠适应性喂养1周后采用随机数表法将大鼠分为对照组(Fen 0 mg/kg)、低剂量Fen染毒组(20 mg/kg)、高剂量Fen染毒组(40 mg/kg),每组8只. 染毒组以玉米油溶解配制成不同浓度的Fen溶液等体积灌胃,灌胃量为每100 g体质量0.5 mL,每日1次,灌胃前后大鼠自由摄食、饮水,连续染毒30 d(大鼠的一个生精上皮周期为12~15 d),对照组给予等量玉米油. 大鼠按12 h白昼的节律调节光照,保持适宜温度(22±2 ℃)和相对湿度(60%±5%). 连续染毒30 d后,将大鼠称质量后麻醉,心脏采集血样,然后迅速分离双侧附睾和睾丸. 采集的血液待其凝固后离心(常温,1 100 r/min,20 min),吸取上清液分装后于-80 ℃保存,用于检测血清雌激素水平;分离的附睾和睾丸迅速称其质量,然后用生理盐水冲洗表面的血污,附睾立即用于精子计数及活力检测,右侧睾丸经液氮速冻,-80 ℃冻存,用于测定睾丸雌激素水平,检测CYP19A1,ERβ,Bax,Bcl-2,Caspase 3及Caspase 9 mRNA和蛋白的表达情况,左侧睾丸用4%甲醛固定后用于病理结构观察和生殖细胞凋亡检测.
-
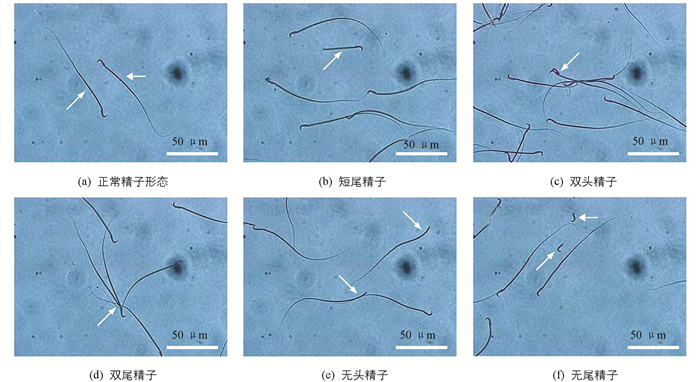
参照Kong等[7]的方法并稍作调整检测雄鼠附睾精子数量及活力情况. 取单侧附睾尾制备精子悬液,将悬液放入含0.1%牛血清白蛋白的Ham 's F-12培养基中,放入37 ℃,5% CO2恒温培养箱中孵育20 min,光学显微镜下(400×)计数400个精子中各级活力(a级:快速直线运动;b级:慢速直线运动;c级:原地打转或运动迟缓;d级:无活动)的精子数,计算各级精子活力和活动率. 于60 ℃水浴中处死精子,用血细胞计数法计数每克附睾中的精子总数. 吸取10 μL精子悬液于载玻片上涂片,凉干,甲醇固定30 min,伊红染液染色10 min,自来水冲洗玻片,凉干后镜检,每个样本计数1 000个精子,统计正常与畸形精子数,计算精子畸形率.
-
取固定于4%甲醛的睾丸组织,经梯度乙醇脱水、浸蜡、石蜡包埋、切片(厚4 μm),每只动物随机选择4张切片,经HE染色,中性树胶封片,然后于光镜下观察其形态学变化,如曲细精管的完整性、生殖细胞的数量等.
-
睾丸组织匀浆制备:用预冷的磷酸盐缓冲液PBS(0.01 mol/L,pH值为7.4)冲洗睾丸组织,去除残留血液,称质量后将组织剪碎. 将剪碎的组织按每克的组织样品加入5 mL的PBS,于冰上充分研磨. 最后将匀浆液离心(4 ℃,1 100 r/min,10 min),取上清分装后于-80 ℃保存用于检测. 操作步骤严格按照E2 ELISA试剂盒说明书进行,取E2标准品、血清或睾丸组织匀浆加入反应孔,加入辣根过氧化物酶(HRP)标记的检测抗体,37 ℃恒温箱温育60 min,洗板,加入反应底物,37 ℃恒温箱避光孵育15 min,加入终止液,测定吸光度(OD值),绘制标准曲线计算浓度.
-
取-80 ℃冻存的睾丸组织100 mg研磨成粉末状,然后采用RNAiso-Plus提取试剂提取样本总RNA,按产品说明书进行实验. 采用分光光度计测定总的RNA浓度,根据光度计260 nm和280 nm处的吸光度比值测定其纯度. 按照RNA反转录试剂盒要求,将所有样本总RNA浓度调整为100 ng/μL,取10 μL进行逆转录,反应条件:37 ℃ 15 min;85 ℃ 5 s;4 ℃保存. 按照Real-time PCR试剂盒说明书要求,取cDNA 2 μL,然后依次加入ddH2O,SYBR Premix Ex Taq(2×),上下游引物(各引物序列见表 1),PCR反应条件:95 ℃ 30 s;40个PCR循环(95 ℃ 5 s;60 ℃ 30 s). 所有反应均设立2个复孔. 记录每个反应管中标本的Ct值,以Gapdh为内参基因,通过2-ΔΔCt法计算各染毒组与对照组的CYP19A1,ERβ,Bax,Bcl-2,Caspase 3及Caspase 9 mRNA的相对表达量.
-
取-80 ℃冻存的睾丸组织60 mg,剪碎后加入裂解液,冰上匀浆并充分裂解30 min,然后离心(4 ℃,13 000 r/min,5 min),取上清. 以BCA法测定总蛋白浓度,每孔以50 μg总蛋白量上样. 12%聚丙烯酰胺凝胶电泳(120 V,1.5 h),恒压转膜(20 V,40~70 min),室温封闭2.5 h,4 ℃过夜孵育一抗,室温孵育二抗1 h,在暗室中用ECL法显影. 目标蛋白相对表达量用目标蛋白的灰度值与内参β-actin的灰度值比值表示.
-
本研究采用末端脱氧核酸转移酶(TdT)介导dUTP缺口末端标记法(TUNEL)检测大鼠睾丸生殖细胞凋亡情况. 该技术的原理是细胞在发生凋亡时,会激活一些DNA内切酶,剪切核小体间的基因组DNA,暴露出3'-OH,然后暴露的3'-OH可在TdT的催化下加上荧光素-12-脱氧三磷酸腺苷(FITC-12-dUTP),而后即可通过荧光显微镜观察生殖细胞的凋亡情况. 检测按试剂盒说明书进行,取睾丸石蜡切片(切片制作同睾丸组织病理形态学检查)经脱蜡水合,滴加蛋白K酶工作液37 ℃作用25 min,滴加平衡液室温平衡20 min,滴加TdT酶工作液37 ℃湿盒避光孵育60 min,滴加DAPI室温避光孵育8 min进行染核,甘油封片,在荧光显微镜下观察拍照,凋亡细胞呈强绿荧光. TUNEL反应阳性细胞计数是在荧光显微镜下(200×)每张切片随机选取4个不同视野进行计数.
-
所有计量资料均以x±s表示,使用SPSS 17.0统计软件,采用单因素方差分析和Duncan法进行多重比较,p<0.05为差异有统计学意义.
1.1. 材料
1.1.1. 主要试剂
1.1.2. 仪器
1.2. 方法
1.2.1. 实验动物与处理
1.2.2. 精子计数、活力及畸形检测
1.2.3. 睾丸组织病理形态学检查
1.2.4. 血清及睾丸雌二醇(E2)检测
1.2.5. Real-time PCR法检测大鼠睾丸CYP19A1,ERβ,Bax,Bcl-2,Caspase 3及Caspase 9的mRNA表达情况
1.2.6. Western Bolt法检测大鼠睾丸CYP19A1,ERβ,Bax,Bcl-2,Caspase 3及Caspase 9的蛋白表达情况
1.2.7. TUNEL法检测睾丸生殖细胞凋亡
1.2.8. 统计学处理
-
如表 2所示,与对照组相比,20 mg/kg和40 mg/kg组大鼠睾丸、附睾质量及体质量均有所降低,但差异无统计学意义,p>0.05.
-
如表 3所示,与对照组相比,20 mg/kg组精子总数、各级精子活力及畸形率差异无统计学意义(p>0.05);40 mg/kg组附睾精子总数、a级活力精子、精子活动率及畸形率(精子畸形情况见图 1)差异有统计学意义(p<0.05或p<0.01).
-
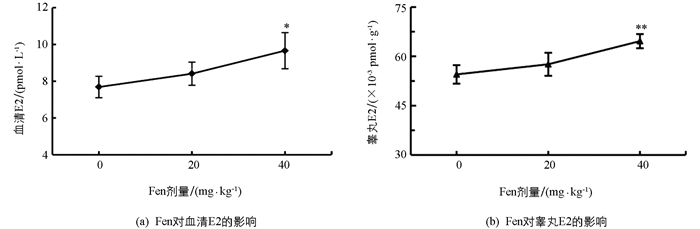
如图 2所示,与对照组相比,20 mg/kg组血清和睾丸中E2水平均未显著增加(p>0.05);40 mg/kg组血清和睾丸中E2水平均显著升高(p<0.05,p<0.01). 提示Fen具有提升雄性体内E2水平的作用,且这种作用随Fen染毒剂量的增加而加强.
-
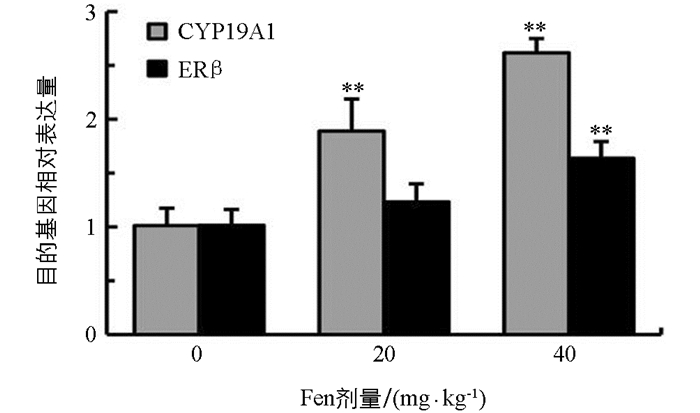
如图 3所示,与对照组相比,雄鼠睾丸CYP19A1和ERβ mRNA表达水平均随染毒剂量增加而上调,CYP19A1 mRNA表达水平在20 mg/kg和40 mg/kg组均极显著上调(p<0.01);ERβ mRNA表达水平在20 mg/kg组差异无统计学意义(p>0.05),在40 mg/kg组差异有统计学意义(p<0.01),提示Fen具有上调雄鼠睾丸CYP19A1和ERβ基因表达的作用.
-
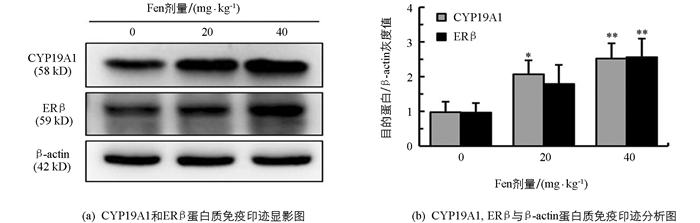
如图 4所示,与对照组相比,雄鼠睾丸CYP19A1和ERβ蛋白表达水平均随染毒剂量增加而增加,CYP19A1在20 mg/kg组和40 mg/kg组差异均有统计学意义(p<0.05,p<0.01);ERβ在20 mg/kg差异无统计学意义(p>0.05),在40 mg/kg组差异有统计学意义(p<0.01),提示Fen具有上调雄鼠睾丸CYP19A1和ERβ蛋白表达的作用.
-
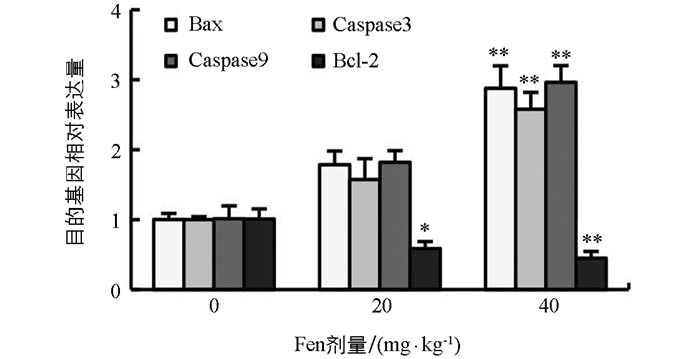
如图 5所示,与对照组相比,雄鼠睾丸Bax,Caspase 3及Caspase 9 mRNA表达水平均随染毒剂量增加而上调,Bcl-2 mRNA表达水平随染毒剂量增加而下调,Bax,Caspase 3及Caspase 9 mRNA表达水平在40 mg/kg组均极显著上调(p<0.01),Bcl-2 mRNA表达水平在20 mg/kg组显著下调(p<0.05),在40 mg/kg组极显著下调(p<0.01).
-
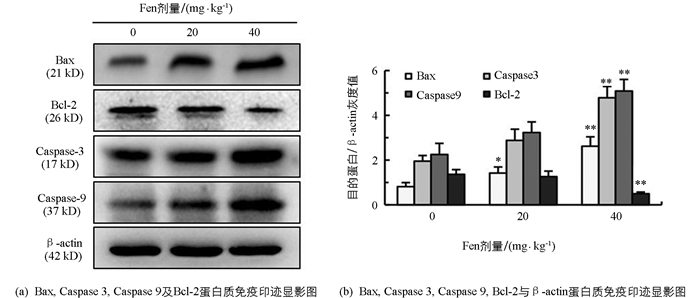
如图 6所示,与对照组相比,雄鼠睾丸Bax,Caspase 3,Caspase 9蛋白表达水平均随染毒剂量增加而上调,Bcl-2蛋白表达水平随染毒剂量增加而下调,Caspase 3,Caspase 9及Bcl-2蛋白表达水平在40 mg/kg组差异有统计学意义(p<0.01);Bax蛋白表达水平在20 mg/kg和40 mg/kg组差异均有统计学意义(p<0.05,p<0.01).
-
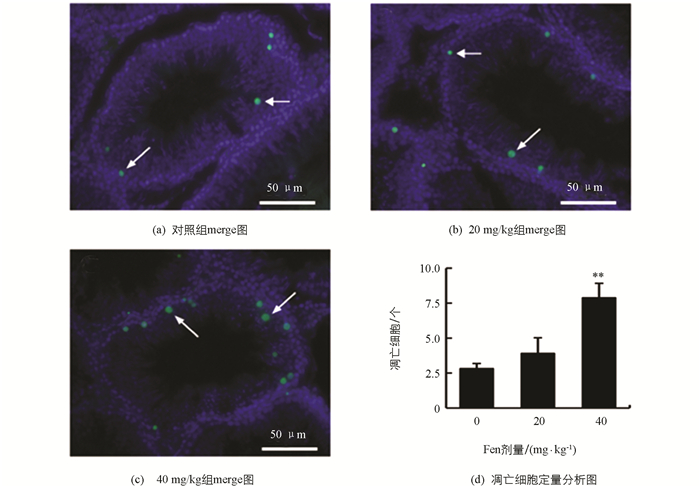
如图 7显示,与对照组相比,Fen染毒组雄鼠睾丸生殖细胞凋亡数量(1个视野中)增加,在40 mg/kg组差异有统计学意义(p<0.01),提示随着Fen染毒剂量的增加,雄鼠睾丸生殖细胞凋亡逐渐增多.
-
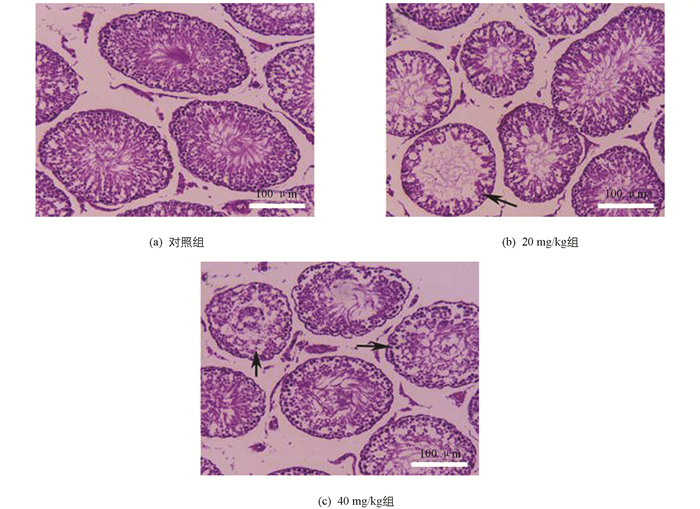
如图 8所示,对照组大鼠曲细精管结构完整,生精细胞数量多,排列紧密;20 mg/kg组大鼠睾丸曲细精管生精细胞数量减少,部分曲细精管出现了较为严重的生精细胞缺失(箭头所示);40 mg/kg组大鼠睾丸曲细精管生精细胞数量进一步减少,可见部分曲细精管生精细胞排列紊乱,生精细胞缺失严重,管腔内精子较少.
2.1. Fen对大鼠睾丸、附睾质量及体质量的影响
2.2. Fen对雄性SD大鼠附睾精子质量的影响
2.3. Fen对雄性SD大鼠血清及睾丸E2水平的影响
2.4. Fen对雄性SD大鼠睾丸CYP19A1和ERβ mRNA表达的影响
2.5. Fen对雄性SD大鼠睾丸CYP19A1和ERβ蛋白表达的影响
2.6. Fen对雄性SD大鼠睾丸Bax,Caspase 3,Caspase 9及Bcl-2 mRNA表达情况的影响
2.7. Fen对雄性SD大鼠睾丸Bax,Caspase 3,Caspase 9及Bcl-2蛋白表达的影响
2.8. Fen对雄性SD大鼠睾丸曲细精管中生精细胞凋亡的影响
2.9. Fen对雄性SD大鼠睾丸病理结构的影响
-
正常雄性生育能力的维持依赖于精子发生的有效进行. 正常有效的精子发生既有赖于睾丸中的生精细胞、支持细胞和间质细胞之间的密切协调,又受到下丘脑-垂体-性腺轴的生殖内分泌调控,同时这两个方面之间还相互协调、相互依存,彼此密不可分. 在生殖内分泌调控中,雄性精子发生主要受黄体生成素介导的睾酮分泌的调控,但雌激素在雄性精子发生过程中也同样起着不可或缺的作用. 研究显示,适量的雌激素在雄性生殖中具有促进精子发生、抑制生殖细胞凋亡、提高精子受精能力等作用[8-10],而雄性体内雌激素的含量过低或过高均会引起雄性精子发生异常以及生殖能力下降[11-12].
在药理学和毒理学中,体质量及脏器系数是一个关键的毒性观察指标. 结果显示,青春期雄性SD大鼠暴露于Fen后,大鼠的体质量及附睾和睾丸的脏器系数均出现减小的趋势,但差异均无统计学意义,这表明本研究中Fen的剂量对雄鼠整体的生长情况并未产生太大的影响.
精子质量是衡量雄性生殖能力的重要指标[13]. 在精子发生的过程中,各级生精细胞对有毒化学物质的作用十分敏感,易受到有毒化学物质的干扰而导致精子的数量减少和活力降低. 本研究的结果表明,Fen染毒后,各染毒组精子数量、精子活力和精子活动率均较对照组降低,精子畸形率较对照组增加,且在40 mg/kg组差异有统计学意义,这与Zhang等[14]的研究结果一致. 这说明Fen对雄性的生殖能力具有损害作用.
雌二醇(E2)是雄性体内最主要的雌激素. 本研究的结果表明,在Fen连续染毒30 d(两个生精上皮周期)后,雄性大鼠血清和睾丸中E2含量均较对照组增高,并且在40 mg/kg组,二者差异均有统计学意义,这表明Fen可促进雄性体内E2的生成,提高雄鼠体内雌激素的水平. Carreau等[11]研究发现,雄性睾丸中雌激素水平过高会引起雄性精子发生异常,使精子质量降低,导致雄性生殖能力下降. 这提示我们Fen可通过增加雄性睾丸中雌激素的水平而损害雄性生殖功能.
雄性体内的E2由雄激素在睾丸中转化而来,芳香化酶(CYP19A1)是这一转化过程中的关键酶. CYP19A1在雄性睾丸间质细胞、生精细胞及精子中均有表达[15]. 以往的研究发现,CYP19A1在雄性体内低表达或过度表达均会引起雄性精子发生异常,使精子数量减少和活力降低,导致雄性生殖损伤甚至不育[6, 12, 16-18],这主要与CYP19A1表达异常致使雄性睾丸内E2合成不足或过量有关[11-12]. 本研究中各染毒组睾丸CYP19A1的mRNA和蛋白表达水平较对照组均显著增加,这与Fen染毒后引起血清和睾丸中E2含量增加的研究结果相一致. 这表明Fen是通过诱导睾丸中CYP19A1的表达,促进雄激素转化为雌激素,从而升高雄性睾丸中雌激素水平的.
雌激素发挥其生理作用需通过其特异性受体(雌激素受体,ERs)的介导. ERs属于激素核受体超家族的成员,其主要包括ERα和ERβ两种亚型,其中ERβ主要分布于成熟雄性大鼠的支持细胞和生精细胞[19, 20],对维持正常雄性生殖能力发挥着十分重要的作用. Delbes等[21]研究发现,敲除小鼠ERβ可促进小鼠生殖母细胞的增殖同时抑制其凋亡;Gould等[22]和Dumasis等[23]发现,ERβ受体激动剂可促进生精细胞的凋亡. 我们的研究结果显示,Fen可诱导大鼠睾丸中ERβ的mRNA和蛋白高表达,并且40 mg/kg组与对照组相比差异有统计学意义(p<0.01). 同时,我们还发现,随着ERβ的表达量升高,雄性大鼠曲细精管发生凋亡的生精细胞比例也逐渐增加,这与Xie等[24]以及Qu等[25]用双酚a、全氟辛烷磺酸等其他环境内分泌干扰物研究的结果相一致. 这些结果提示我们,Fen可能通过诱导雄鼠睾丸中ERβ的表达,过度增强ERβ信号作用,而促进生精细胞的凋亡,使曲细精管中生精细胞数量和管腔内精子数量减少,但ERβ引起生精细胞凋亡的机制尚不清楚.
线粒体凋亡途径是细胞最重要的凋亡途径之一,在各种外源性病理刺激条件下,Bcl-2家族中的促凋亡蛋白(如Bax,Bad等)发生去磷酸化,与线粒体膜上的抗凋亡蛋白(如Bcl-2,Bcl-XL等)结合,而开启线粒体膜通透性转换孔,导致凋亡相关因子,如细胞色素C、凋亡诱导因子等从线粒体内释放进入胞浆,激活Caspase家族的凋亡启动者Caspase 9,Caspase 9激活后能引起Caspase级联反应,最终激活凋亡执行者Caspase 3,而引起细胞凋亡[26-28]. 我们的研究结果显示,Fen染毒上调了大鼠睾丸中促凋亡基因Bax,Caspase 3及Caspase 9 mRNA和蛋白的表达水平,同时抑制了抗凋亡基因Bcl-2 mRNA和蛋白的表达. 这些结果似乎提示我们,Fen诱导的雄鼠睾丸ERβ过度升高可能与线粒体凋亡途径的激活之间存在着某种联系,但具体机制还有待进一步研究.
综上所述,我们猜测Fen通过上调雄性大鼠睾丸中CYP19A1和ERβ的表达,致使睾丸E2水平升高的同时,又过度增强E2-ERβ信号作用,后者通过某种机制激活线粒体凋亡途径,从而引起生殖细胞的过度凋亡,精子发生异常,精子数量和活力降低,畸形精子增多,最终导致雄性大鼠的生殖损伤. 然而Fen是通过何种机制上调雄性大鼠睾丸中CYP19A1和ERβ的表达水平以及ERβ的高表达是否与生殖细胞线粒体凋亡途径的激活有关,这些问题都还有待今后进一步明确.






 DownLoad:
DownLoad: