-
开放科学(资源服务)标志码(OSID):

-
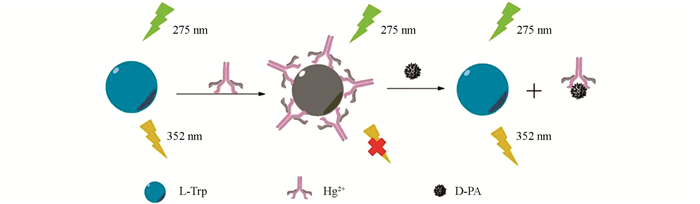
青霉胺(3,3-二甲基半胱氨酸,PA)是青霉素的代谢产物,也是一种含巯基氨基酸[1]. 同时青霉胺也是一种具有药理作用的手性化合物,用于治疗多种疾病,如威尔逊氏病、类风湿性关节炎、硬皮病及重金属中毒等[2]. 青霉胺作为手性化合物,存在D-青霉胺(D-PA)和L-青霉胺两种对映异构体,它们的药效及毒理作用差别很大,其中L-PA有一定毒副作用,只有D-PA有治疗疾病的作用. 世界卫生组织已经将D-PA列为基础医疗中的必备药品. 因此,建立简单、快速、高灵敏、高选择检测D-PA的方法具有重要意义. 目前报道的检测D-PA的主要方法有电化学发光[3]、电化学法[4]、分光光度法[5]、HPLC法[6]、圆二色性法[7]和离子色谱法[8]. 此外,荧光光谱法相比于其他方法更简单、高效,且灵敏度更高. 近年来,已有较多测定D-PA的荧光法报道[9-11],但是有的荧光探针合成步骤复杂繁琐,限制了方法的使用. 本研究利用一种简单易得的荧光探针来检测D-PA. 研究发现,L-色氨酸(L-Trp)在352 nm处有很强的荧光发射峰,随着Hg2+的加入,L-Trp荧光被猝灭. 接着加入D-PA,L-Trp的荧光得到恢复,据此建立了一种“开-关”模型来检测D-PA,其反应机理见图 1.
HTML
-
Hitachi F-2500型荧光分光光度计(日立科学仪器有限公司,日本);UV-2450型紫外-可见分光光度计(岛津公司,日本);pHSJ-4F酸度计(上海仪电科学仪器股份有限公司,上海).
L-Trp、D-PA(阿拉丁试剂有限公司,上海);HgCl2(四川科伦医药贸易有限公司,成都);Britton-Robinson缓冲溶液:2.71 mL 85% 磷酸(重庆北碚化学试剂厂,重庆),2.36 mL冰醋酸(重庆川东化工有限公司,重庆)和2.47 g硼酸(重庆北碚化学试剂厂,重庆)溶于1.0 L蒸馏水中,用0.2 mol/L NaOH调节不同pH值. 实验过程中所用水均为超纯水.
-
在10.0 mL的比色管中依次加入pH=4.8的BR缓冲溶液1.0 mL,0.5 mL 4.0 × 10-4 mol/L L-色氨酸,0.5 mL 1.0 × 10-2 mol/L Hg2+和一系列不同浓度的D-PA,用蒸馏水定容至刻度后摇匀,在室温下静置15 min. 在荧光分光光度计上以λex=275 nm激发,进行波长扫描,记录体系的荧光光谱,并在波长352 nm处测定样品和试剂空白的荧光强度,ΔF=F-F0. 狭缝宽度为10 nm.
1.1. 实验仪器与材料
1.2. 实验方法
-
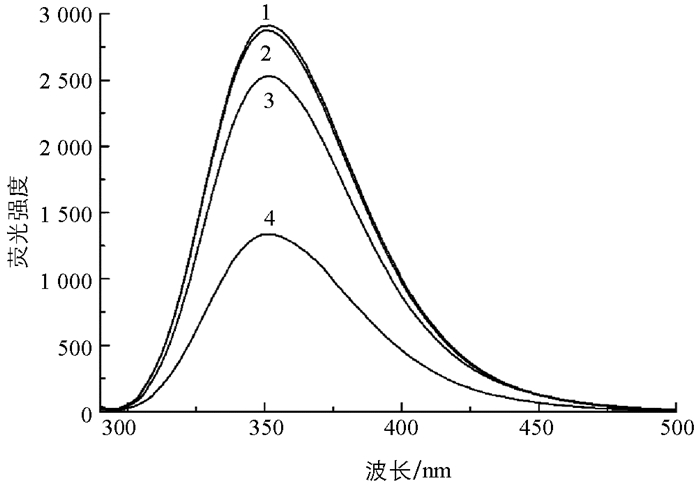
以最大激发波长275 nm对L-Trp溶液进行荧光光谱扫描,发现其最大荧光峰位于352 nm处(图 2中曲线1),而Hg2+和D-PA均无荧光. L-Trp与D-PA溶液混合,L-Trp的荧光强度几乎没有变化(图 2中曲线2). 然而,在一定浓度的L-Trp中加入适量Hg2+后,由于Hg2+与L-Trp结构中的羧基结合,使得L-Trp在352 nm的荧光猝灭[12](图 2中曲线4),接着向溶液中加入与Hg2+结合能力更强的D-PA,L-Trp被释放出来,溶液荧光逐渐得到恢复(图 2中曲线3).
-
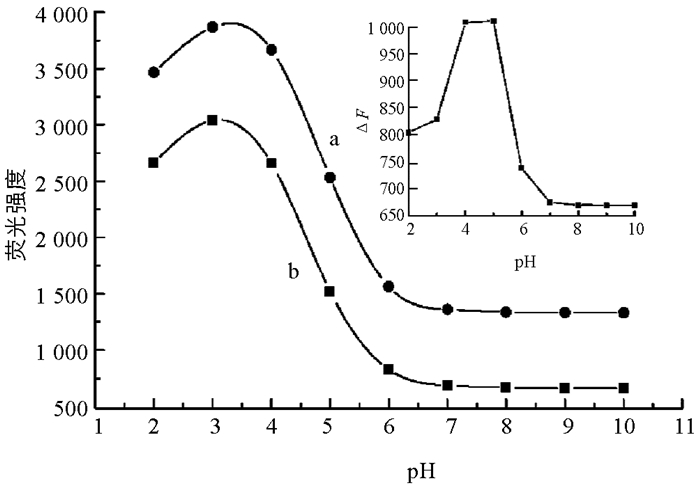
实验探究了不同pH值的BR缓冲溶液对反应体系的影响. 由图 3可知,L-Trp-Hg2+和L-Trp-Hg2+-D-PA两个反应体系的荧光强度在pH=2.0~3.1范围逐渐增强,pH=3.1~7.2之间荧光逐渐减弱,但荧光恢复值(ΔF)在pH=4.0~5.0时最大(图 3插图). 因此本实验选用pH=4.8的BR溶液为反应介质.
-
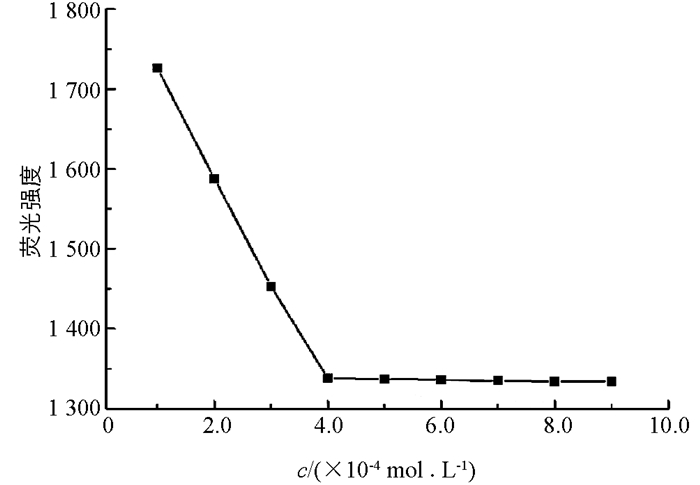
L-Trp作为一种常见天然氨基酸,已有文献报道Hg2+可以与羧基螯合从而猝灭L-Trp的荧光[13]. 如图 4所示,在Hg2+浓度为4.0×10-4 mol/L时,L-Trp荧光猝灭程度最大,且随着Hg2+继续加入,荧光强度保持不变. 故本实验选择5.0×10-4 mol/L为Hg2+后续实验浓度.
-
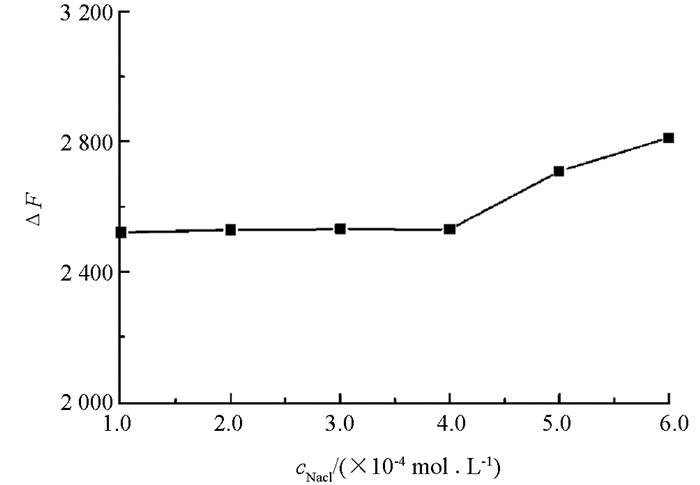
研究了离子强度对三元反应体系的影响(图 5),当NaCl浓度低于4.0×10-4 mol/L时,反应体系荧光强度较稳定;当NaCl浓度高于4.0×10-4 mol/L时,荧光强度有所增加,因此,在实际样品检测过程中要注意控制离子强度,避免高浓度盐类的引入. 此外,实验发现L-Trp的荧光在15 min恢复到最大值并且在1 h内保持稳定,因此选择15 min为反应时间.
-
为了考察该方法的选择性,在优化的实验条件下,探究了20种常见物质对测定D-PA的影响. 当共存物质引起的荧光强度改变在相对误差±5%之内,通常认为不会对检测造成影响. 由表 1可知,常见的金属离子、氨基酸、糖类对D-PA的检测几乎没有影响,表明本法具有较好的选择性,可以应用于实际样品的检测.
-
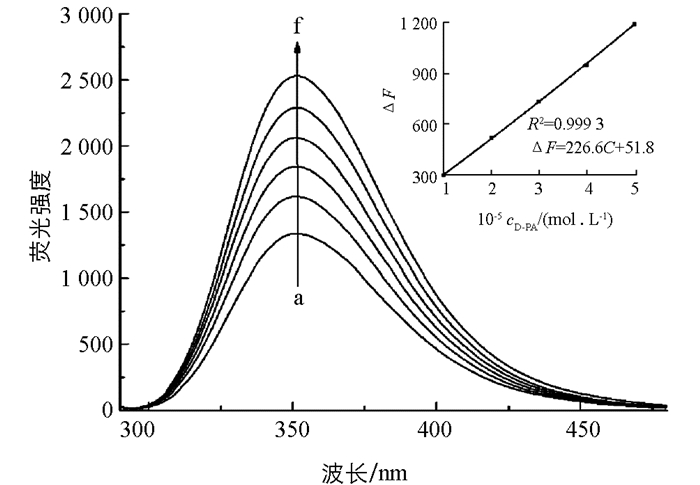
按照前述实验方法,测定不同D-PA浓度下反应体系的荧光强度. 如图 6所示,随着D-PA的加入,L-Trp的荧光逐渐恢复,荧光恢复程度(ΔF)与D-PA浓度在0.44~50.0 μmol/L范围内呈现良好的线性关系(图 6插图),线性回归方程为ΔF=226.6 C+51.8,相关系数为0.999 3,检出限(3σ/K)达0.13 μmol/L. 表 2列出了本法与其他方法测定D-PA的比较,由此可以看出,本法灵敏度高,操作简单,更具有实用价值.
-
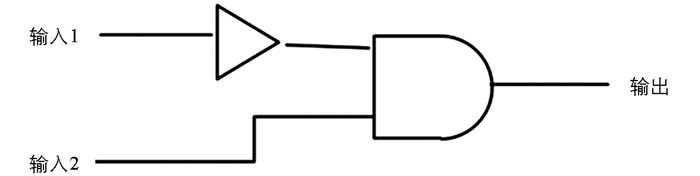
分子传感器与目标分析物协同作用,对反应条件进行信息处理,将一个或多个反应条件作为输入信号,荧光信号的改变作为输出信号[14]. 基于荧光响应可以通过Hg2+和D-PA的加入来回切换,体系荧光强度进入“on-off-on”模式,为此构建了IMPLICATION逻辑门[15]. 以L-Trp作为一种逻辑门装置,设定Hg2+(输入1)和D-PA(输入2)为输入信号,352 nm处荧光强度为输出信号,0和1分别代表荧光猝灭和未猝灭. 无信号输入(0,0)或只有D-PA输入(0,1),352 nm处荧光吸收很强,输出为1;单独Hg2+输入(0,1)时,荧光猝灭,输出为0. 当两者同时输入(1,1)时,L-Trp荧光恢复,输出为1. 表 3为逻辑门对应的真值表,图 7为荧光输出IMPLICATION逻辑图.
-
为了评价本方法的适用性和有效性,将本方法用于青霉胺药片中D-PA含量检测. 随机选10片药片(上海信谊药厂有限公司),研细. 称取相当于0.25片质量的样品,溶解、过滤、除去不溶物,最后定容至500.0 mL容量瓶中,备用. 吸取0.5 mL待测液于10.0 mL比色管中进行检测, 结果见表 4. 通过标准加入法测得回收率和相对标准偏差分别为96.0%~103.8% 和2.8%~4.0%,表明本方法具有较高的准确度和较好的重复性,可以用于实际样品的测定.
2.1. 荧光光谱
2.2. 反应条件优化
2.2.1. pH值的影响
2.2.2. Hg2+浓度的影响
2.2.3. 离子强度和反应时间的影响
2.3. 方法的选择性
2.4. 标准曲线
2.5. 逻辑门的模拟应用
2.6. 实际样品的测定
-
利用Hg2+与D-PA之间的强螯合作用,使得Hg2+脱离L-Trp,L-Trp荧光得到恢复,从而设计了分子逻辑门,并建立了D-PA的荧光检测新方法. 本方法相较于其他方法,简单易行、选择性好、灵敏度高,无需精密的仪器和复杂的合成步骤,适用于青霉胺药片含量的测定.






 DownLoad:
DownLoad: