-
开放科学(资源服务)标识码(OSID):

-
洞穴鱼类栖息于洞穴水体中,演化出了很多适应洞穴环境的特征[1-2]. 由于视觉功能对于在黑暗的洞穴环境中感知信息不再重要,很多洞穴鱼类的视觉器官退化,视觉功能丧失,以利于节约能量、适应食物资源有限的洞穴环境[2-6]. 相反,非视觉功能对洞穴鱼类更为重要,可能补偿性增强,从而弥补因视觉退化而损抑的感觉功能. 从生物能量学的角度分析,由于神经系统及感觉器官的能量需求占比很高,且各感觉器官间的可分配能量有限,因而视觉与非视觉感觉可能存在能量和功能权衡[5, 7],因此,可推测洞穴鱼类视觉退化节约的能量可能补偿了非视觉感觉功能的需求,如洞穴鱼类通常味蕾较多,嗅板较大,神经丘较多,因而嗅觉和侧线机械感觉功能较强[2, 8-9]. 听觉也是鱼类的感觉方式之一,由于水的物理特征有利于声音传播,因此听觉功能对鱼类执行觅食、避敌及种内交流等行为活动有明显意义[10-12]. 有关洞穴鱼类听觉的研究甚少,仅有的研究发现,洞穴鱼类南方盲鮰鲈(Typhlichthys subterraneus)和洞鲈(Amblyopsis spelaea)与非洞穴鱼类阿氏亮鮰(Forbesichthys agassizii)的听觉灵敏性相近[13]. 由于已有的研究资料有限,且在进行比较时未考虑物种间系统发育关系的影响,洞穴鱼类的听觉功能究竟如何仍值得研究.
鱼类的听觉功能主要由内耳执行[14],除了内耳,一些鱼类的鳔也可作为压力位移传感器参与听觉,其体积受声压刺激而变化,引起鳔膜振动,该振动波通过特殊的结构传至内耳,从而放大声音信号,可提高听觉的灵敏性[15-16]. 鳔对声波振幅的放大能力取决于鳔室的体积及其至内耳的距离(鳔耳距)等形态指标[17],但这些形态指标是否影响鱼类的听觉灵敏性目前尚无定论. 在很多鲇类中,鳔越大的物种,其听觉灵敏性越高[18],而在棘甲鲇科鱼类中恰恰相反[19]. 鳔耳距越小的鱼类,其听觉灵敏性越高[20-23],在石首鱼科鱼类中,物种间的鳔耳距差异明显,但听觉灵敏性很接近[24]. 可见,鳔的形态与鱼类听觉灵敏性的关系究竟如何还需要更多的研究资料加以验证.
听觉灵敏性常表征为听觉阈值,即能听到的最小声压级. 听觉阈值越低,听觉灵敏性越高. 鱼类的听觉阈值通常可采用声音惊吓导致的电生理或行为反应进行测量[25-27]. 相对而言,鱼类对声音刺激的行为反应更易观测. 鱼类受到声音刺激后,身体肌肉极速收缩,产生快速的体态变化和运动行为变化,即惊吓反应,如“C-start”反应[28]、趋流游泳[29]及频繁活动[30]等,观测这些行为变化可直观高效地评估鱼类的听觉灵敏性[28, 31-33].
由于人类活动的干扰,洞穴水环境条件变化明显[34],有关洞穴鱼生理生态学研究的重要性日趋凸显. 高原鳅属(Triplophysa)鱼类隶属鲤形目(Cypriniformes),条鳅科(Nemacheilidae),主要分布于青藏高原及其邻近地区,该属鱼类中有很多物种为洞穴鱼类,视觉器官退化[35-36]. 有关该属一些洞穴鱼类的形态结构、能量代谢、系统演化及比较基因组研究已有报道[37-40],但它们的听觉功能如何仍需继续研究. 高原鳅属鱼类为骨鳔鱼类,鳔的形态较独特,前室分为左、右两个球形侧室,由骨质鳔囊不完全包裹,骨质鳔囊两侧均有侧孔,大小因物种而异[35, 41-42]. 这些特征是否与听觉灵敏性有关尚不清楚. 本研究以高原鳅属3种洞穴鱼类佳荣盲高原鳅(T. jiarongensis)、长须盲高原鳅(T. longibarbata)和玫瑰高原鳅(T. rosa)与3种非洞穴鱼类鼻须高原鳅(T. nasobarbatula)、洞腮高原鳅(T. dongsaiensis)和贝氏高原鳅(T. bleekeri)为对象,通过观测行为反应评估听觉阈值,并测量鳔的形态指标,在控制种间系统发育关系的基础上,探究洞穴鱼类与非洞穴鱼类的听觉功能差异及其与鳔形态结构的关系.
HTML
-
洞穴鱼类佳荣盲高原鳅(n=18)、长须盲高原鳅(n=9)和玫瑰高原鳅(n=18)分别采自贵州省荔波县佳荣镇拉滩村水井湾溶洞、洞塘乡老爷洞和重庆市武隆区火炉镇梦冲塘. 非洞穴鱼类鼻须高原鳅(n=23)、洞腮高原鳅(n=23)和贝氏高原鳅(n=24)分别采自贵州省荔波县洞塘乡洞得上洞、黎明关水族乡尧所村洞腮组喀斯特地下河和重庆市巫溪县大宁河. 将在野外采集到的鱼类运回西南大学鱼类实验室,在装有天然地下水的鱼缸中避光暂养恢复3 d,每天18:00用红线虫饱足喂食1次. 采用恒温器(JRB-250,舟山阳光有限公司)将水温维持在15±0.5 ℃(接近采样来源水温);采用气泵(BKL-902,上海崇特宠物产品有限公司)充气将溶解氧保持在99%以上. 实验鱼的利用遵照西南大学伦理委员会的规定(IACUC-20210119-01),也符合采集地渔业管理的规定. 在末次喂食后24 h,开始观测实验鱼的听觉行为反应,评估听觉阈值.
-
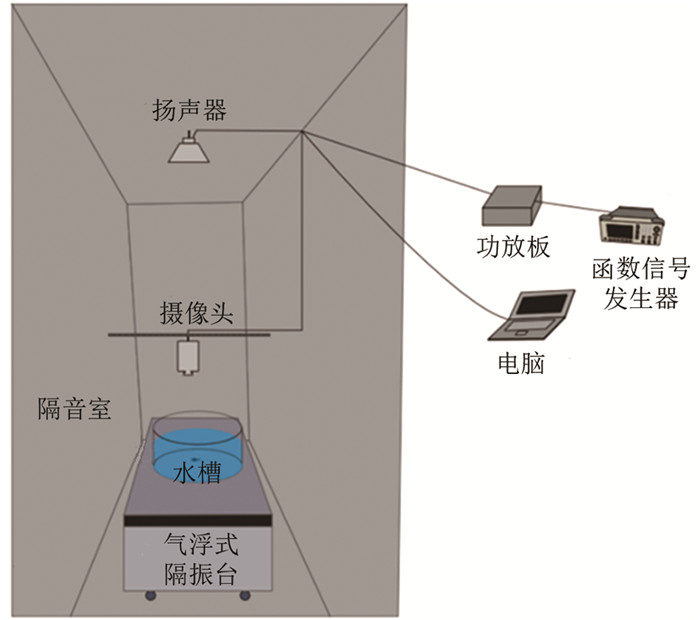
测定听觉阈值采用的行为反应观测装置参照Kenyon等[25]的研究,经改良后自制(图 1). 采用磨砂亚克力板制成的圆形观测水槽(直径35 cm,高15 cm),置于气浮式隔振台上(HGPT-TB456B,北京衡工仪器有限公司),以尽量避免外源性振动干扰. 在观测水槽上方1 m处置有扬声器(两种功率:60 W和3 W),可接收由函数信号发生器(UTG1005A,优利德)发出并经功放板放大的正弦波信号,发出不同频率(Hz)和声压(dB)的声音. 在观测水槽正上方1 m处置有摄像头(C270,Logitech Webcam Pro),并连接到电脑,由摄像头录像大师软件(天津市中格科技有限公司)控制视频拍摄. 由4盏4 W的LED灯管提供散射光源,以保证视频拍摄过程中光照均匀. 采用由2层隔音海绵(厚度均为3 cm)及中间1层隔音板(厚度为0.9 cm)制成隔音室(长×宽×高=80 cm×80 cm×130 cm),尽量避免外源性声音干扰.
将禁食24 h的实验鱼单尾放入观测水槽中,水深7 cm,水温15±1 ℃,适应30 min后,开始采用不同频率和声压的声音进行刺激,观测实验鱼的行为反应. 共设置8个声音频率(50,100,200,400,800,1 600,3 200,6 400 Hz),声压均从环境噪音(26±0.2 dB)的基础上递增,以发生惊吓反应的声压作为实验鱼听觉阈值. 每次声音刺激时长为15 s,间隔5 min进行下一次声音刺激,以使实验鱼的行为恢复正常[30]. 在较低频率(50~400 Hz)下声压每次递增1 dB,在较高频率(800~6 400 Hz)下声压每次递增5 dB. 在实验过程中,使用声级仪(AWA5688,杭州爱华仪器有限公司)实时监测环境噪音. 惊吓反应的判定标准参照前人[30, 32]研究,采用“C-start”行为(头与尾夹角从180°变为45°以下)、活动频次,并增加了变速(15 s内平均速度增、减10%及以上)、转向(15°及以上)和扭动(2次及以上)等行为反应指标. 每次声音刺激后,当实验鱼表现出上述任一行为反应时,再递增1次声压,若仍有反应,则确定递增前的声压级为实验鱼在该频率下的听觉阈值. 若实验鱼听觉阈值超过实验装置的观测上限(105 dB),则在观测上限的基础上增加5 dB作为听觉阈值.
使用狸窝全能视频转换器软件(深圳市漠野软件有限公司)将拍摄的视频转换为每秒10帧的AVI格式,使用ImageJ(National Institutes of Health)分析每尾鱼在声音刺激前、后各15 s的体态及坐标位置(x,y),用以确定惊吓反应. 根据坐标位置计算实验鱼每秒的运动路径(cm),求得声音刺激开始前和开始后15 s的平均速度(cm/s).
-
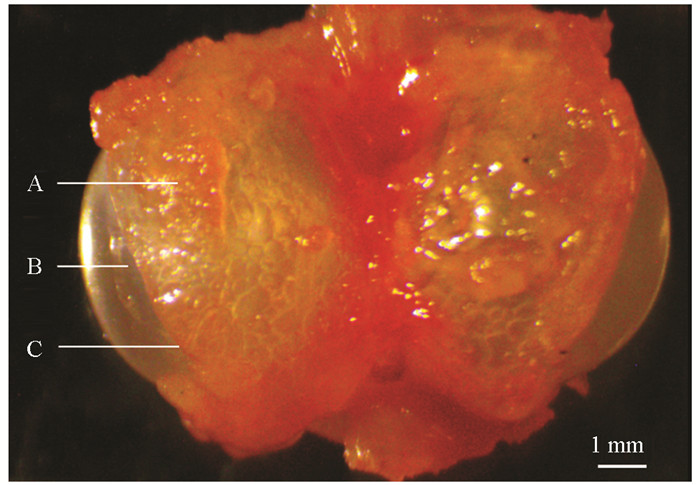
听觉行为反应观测结束后,将实验鱼放回鱼缸中恢复24 h,用麻醉剂(0.9 g/L NaHCO3,0.45 g/L MS-222)麻醉20~40 s,待实验鱼失去活动能力后,称体质量,精确到0.01 g;测量体长,精确到0.1 cm;然后解剖实验鱼,用电子游标卡尺测量鳔耳距(Swim bladder-inner ear distance,Dbe),即鳔的前端到内耳的距离,精确到0.01 mm;取出完整的鳔,测量左侧骨质鳔囊开孔(鳔孔)的长径(Major axis of hole,Lah)和短径(Minor axis of hole,Lih),精确到0.01 mm(图 2),用以计算鳔孔面积(Hole area of swim bladder bony capsule,Sh):
用镊子小心去除左侧鳔室外的骨质鳔囊,使用电子游标卡尺测量左侧鳔室的高度(Height of swim bladder,Lhs),精确到0.01 mm. 使用连接数码显微图像分析系统的体式显微镜(OPTPro 3000,重庆奥特光学仪器有限责任公司)拍测左侧鳔室的长径(Major axis of swim bladder,Las)和短径(Minor axis of swim bladder,Lis),用以计算鳔室体积(Volume of swim bladder,Vs):
为评估鳔孔的相对大小,计算了鳔孔面积与鳔室体积的比值,即孔室比(Ratio of hole area of swim bladder bony capsule to volume of swim bladder,Sh/Vs).
-
采用软件Excel 2010(Microsoft Corporate,Redmond,WA,USA)进行常规数据计算,然后采用软件R进行后续统计分析. 采用线性模型(lm)分析各物种的听觉阈值及形态指标与体长的关系,若相关则采用所有实验鱼的平均体长进行矫正,若不相关,则采用原始数值[43]. 分析的数据先采用fitdistrplus包中的fitdist检验数据分布类型,根据分布类型选择模型及连接函数[44]. 本研究中,听觉阈值为重复测量,即每一实验鱼个体均经历了所有频率下的观测,且各种鱼的听觉阈值均符合gamma分布,因此采用lme4包中的广义线性混合模型(glmer)分析听觉阈值随声音频率变化的种内变化及种间差异,glmer采用log连接函数[45].
种内分析时,为分析听觉阈值随声音频率的变化,以实验鱼个体编号为随机效应,声音频率为固定效应构建模型;为分析听觉阈值与鳔各形态指标的关系,以实验鱼个体编号为随机效应,声音频率和鳔各形态指标为固定效应构建模型.
种间分析时,为比较种间听觉阈值的差异,先采用重复测量方差分析整体是否受声音频率和物种以及两者交互项的影响;而后以实验鱼个体编号为随机效应,声音频率和物种及两者的交互项为固定效应构建模型. 采用单因素方差分析和Tukey's HSD检验分析鳔形态指标的种间差异以及各频率下听觉阈值的种间差异[46-47]. 采用Rmisc包中的summary SE函数计算出各物种在各频率下听觉阈值的均值及各物种形态指标的均值[48]. 采用phytools包中的系统发育方差分析(phyl ANOVA)比较洞穴鱼类与非洞穴鱼类的听觉阈值及形态指标差异[49]. 采用caper包中的系统发育广义最小二乘法(Phylogenetic Generalized Least Squares,PGLS)分析对数化后听觉阈值与形态指标的种间关系[50]. 系统发育方差分析和系统发育广义最小二乘法采用的系统发育关系和分支长度参照Zhang等[51]的方法,数值表示为x±s,显著水平为p<0.05. 采用ggplot2包中的ggplot函数绘图[52].
1.1. 实验鱼的来源
1.2. 听觉阈值
1.3. 鳔的形态
1.4. 统计分析
-
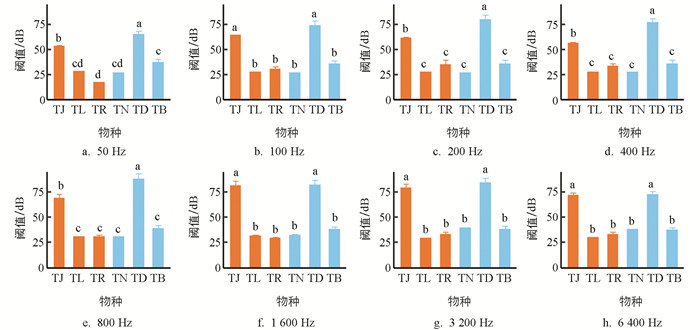
由图 3可知,听觉阈值受物种(F=59.970,p<0.001)、频率(F=50.390,p<0.001),以及二者的交互作用(F=9.050,p<0.001)的影响. 洞腮高原鳅(TD,64~88 dB)和佳荣盲高原鳅(TJ,53~81 dB)的听觉阈值高于其余4种鳅;后4种鳅中,贝氏高原鳅(TB,35~38 dB)高于除鼻须高原鳅(TN,27~40 dB)外的2种鱼;长须盲高原鳅(TL,27~31 dB)、鼻须高原鳅和玫瑰高原鳅(TR,17~35 dB)差异无统计学意义. 听觉阈值随声音频率的变化也因物种不同而不同,随声音频率增高,佳荣盲高原鳅(t=4.139,p<0.001)、玫瑰高原鳅(t=2.265,p=0.024)、鼻须高原鳅(t=12.420,p<0.001)和贝氏高原鳅(t=2.181,p=0.029)的听觉阈值有不同程度的增高,而长须盲高原鳅(t=1.435,p=0.151)和洞腮高原鳅(t=0.485,p=0.628)的听觉阈值无明显线性变化.
各频率下,洞穴鱼类和非洞穴鱼类的听觉阈值均值分别为33~47 dB和43~54 dB. 系统发育方差分析表明,在各频率下,洞穴鱼类和非洞穴鱼类间的听觉阈值差异无统计学意义(50 Hz:F=0.421,p=0.652;100 Hz:F=0.060,p=0.852;200 Hz:F=0.107,p=0.819;400 Hz:F=0.194,p=0.746;800 Hz:F=0.164,p=0.765;1 600 Hz:F=0.021,p=0.917;3 200 Hz:F=0.099,p=0.802;6 400 Hz:F=0.060,p=0.868).
-
观察发现,玫瑰高原鳅的鳔膨大至体表,在身体左右两侧皮下形成明显的囊泡状隆起,鳔侧室呈椭球形,仅少部分被骨质鳔囊包裹,因此鳔孔大(图 2). 鼻须高原鳅的鳔侧室也呈椭球形,骨质鳔囊包裹程度不高,鳔孔也较大. 其余4种高原鳅的鳔侧室均呈球形,且较小,被骨质鳔囊包裹较多,鳔孔较小.
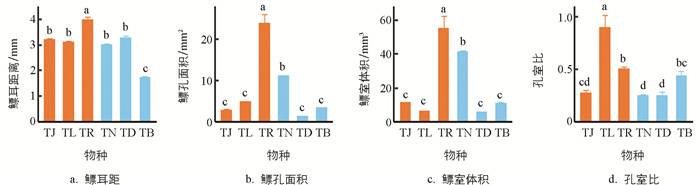
鳔耳距(F=73.650,p<0.001)、鳔孔面积(F=86.760,p<0.001)、鳔室体积(F=43.250,p<0.001)和孔室比(F=19.670,p<0.001)的种间差异均有统计学意义. 玫瑰高原鳅(TR)的鳔耳距(3.96±0.14 mm)大于其余5种鱼;洞腮高原鳅(TD,3.27±0.09 mm)、佳荣盲高原鳅(TJ,3.20±0.07 mm)、长须盲高原鳅(TL,3.08±0.09 mm)和鼻须高原鳅(TN,2.99±0.06 mm)差异无统计学意义,均大于贝氏高原鳅(TB,1.70±0.08 mm)(图 4a).
玫瑰高原鳅(TR)的鳔孔面积(23.89±2.28 mm2)大于其余5种鱼;后5种鱼中,鼻须高原鳅(TN)的鳔孔面积(10.97±0.29 mm2)大于其余4种鱼,依次为长须盲高原鳅(TL,4.72±0.26 mm2)、贝氏高原鳅(TB,3.22±0.13 mm2)、佳荣盲高原鳅(TJ,2.69±0.23 mm2)和洞腮高原鳅(TD,1.28±0.13 mm2),该4种鱼间差异无统计学意义(图 4b).
玫瑰高原鳅(TR)的鳔室体积(54.79±7.92 mm3)也大于其余5种鱼;后5种鱼中,鼻须高原鳅(TN)的鳔室体积(41.17±0.79 mm3)大于其余4种鱼,其余4种鱼的鳔室体积差异无统计学意义,从大到小依次为佳荣盲高原鳅(TJ,11.12±0.71 mm3)、贝氏高原鳅(TB,10.78±0.82 mm3)、长须盲高原鳅(TL,6.01±0.60 mm3)和洞腮高原鳅(TD,5.36±0.54 mm3)(图 4c).
长须盲高原鳅(TL)的孔室比(0.89±0.13)大于其余5种鱼;后5种鱼中,玫瑰高原鳅(TR)的孔室比(0.50±0.02)大于除贝氏高原鳅(TB,0.43±0.05)外的其余3种鱼,且差异有统计学意义;贝氏高原鳅与佳荣盲高原鳅(TJ,0.27±0.03)差异无统计学意义,大于其余2种鱼;佳荣盲高原鳅、洞腮高原鳅(TD,0.25±0.04)和鼻须高原鳅(TN,0.24±0.02)3种鱼间差异无统计学意义(图 4d).
系统发育方差分析表明,鳔的各形态指标在洞穴鱼类与非洞穴鱼类间差异无统计学意义(鳔耳距:F=1.866,p=0.333;鳔孔面积:F=0.512,p=0.599;鳔室体积:F=0.065,p=0.858;孔室比:F=1.658,p=0.339).
-
在种内,听觉阈值与鳔形态的关系因物种及指标不同而不同,各物种的听觉阈值只与部分鳔形态指标有关. 在佳荣盲高原鳅中,与鳔耳距显著负相关(t=-2.202,p=0.028);在玫瑰高原鳅中,与鳔孔面积极显著正相关(t=4.392,p<0.001);在洞腮高原鳅中,与鳔室体积显著负相关(t=-2.431,p=0.015);而在贝氏高原鳅中,与鳔孔面积(t=-48.748,p<0.001)和孔室比(t=-69.940,p<0.001)呈极显著负相关(表 1).
系统发育广义最小二乘法分析表明,在种间,听觉阈值仅与鳔孔面积和孔室比有关,且因声音频率不同而不同. 听觉阈值均值与鳔孔面积均值在50 Hz(t=-6.297,p=0.003)和800 Hz(t=-2.790,p=0.049)下显著负相关;听觉阈值均值与孔室比均值在1 600 Hz(t=-3.053,p=0.038),3 200 Hz(t=-4.083,p=0.015),6 400 Hz(t=-3.562,p=0.024)下显著负相关(表 2).
2.1. 听觉阈值
2.2. 鳔的形态指标
2.3. 听觉阈值与鳔形态的关系
-
50~6 400 Hz下,洞腮高原鳅和佳荣盲高原鳅的听觉阈值较高,分别为64~88 dB和53~81 dB,与前人采用相同方法测得的鲤(Cyprinus carpio)(57~122 dB,105~3 010 Hz)和鲫(Carassius auratus)(65~125 dB,100~3 000 Hz)的阈值大致相当[26-27]. 相比而言,本研究中的其余4种高原鳅的听觉阈值明显较低,贝氏高原鳅为35~38 dB,长须盲高原鳅为27~31 dB,鼻须高原鳅为27~40 dB,玫瑰高原鳅为17~35 dB(图 3),提示这4种高原鳅的听觉较灵敏.
本研究中洞穴鱼类与非洞穴鱼类高原鳅的听觉阈值及鳔的形态指标差异均无统计学意义(图 3,图 4). 前人的研究发现,与非洞穴鱼类阿氏亮鮰相比,洞穴鱼类南方盲鮰鲈和洞鲈的听觉灵敏性相近[13],本研究在控制物种间系统发育关系的潜在干扰后仍得到类似的结果. 这提示尽管洞穴鱼类眼退化,但听觉功能无明显的适应性增强. 可能的解释是,在黑暗寂静的洞穴环境中,听觉对于资源的获取无明显意义. 洞穴鱼类几乎没有被捕食的压力,其食物来源主要有水生无脊椎动物、外来有机物、蝙蝠的粪便及尸体等[37, 53]. 觅食活动需要感知水生无脊椎动物游泳造成的水体波动,以及水体中溶蚀的有机物[54-55]等,因此更需要化学感觉和侧线机械感觉发挥功能,例如,墨西哥丽脂鲤(Astyanax mexicanus)洞穴型的嗅觉和侧线机械感觉比地表型灵敏[9, 56-58].
-
一些研究推测,鱼类的听觉灵敏性可能与鳔的形态结构有关,尤其是鳔的体积和鳔耳距[18-19, 23],分别影响对声波的放大和传导[17]. 也有研究发现,鳔耳距不同的物种间听觉阈值无明显差异[24]. 本研究中,6种鱼的听觉阈值与鳔室体积及鳔耳距间存在一定的种间差异,但所有频率下种间的听觉阈值与鳔室体积和鳔耳距均不相关(表 2). 这表明,鳔较大、鳔耳距较小的物种,不一定具有较敏感的听觉. 种内分析也表明,鳔室体积和鳔耳距对鱼类的听觉功能影响有限,仅在少数鱼类中有影响(表 1).
本研究还发现,在50 Hz和800 Hz下,种间的听觉阈值与鳔孔面积显著负相关;在1 600,3 200,6 400 Hz下,与孔室比显著负相关(表 2),表明在这些频率下,骨质鳔囊侧孔较大的鱼类具有较灵敏的听觉. 由于这些高原鳅的骨质鳔囊侧孔紧贴皮肤,利于将水的压力变化传到鳔,再由韦伯氏器传到内耳[35]. 此外,侧孔的相对面积越大,可通过的能量越多[59],因而传导的声音信号越强,听觉越灵敏.
本研究还观察到一个有趣的现象,玫瑰高原鳅的鳔膨大,在体表左右两侧呈明显的隆起. 尽管有研究推测,鳔前室膨大与高原鳅属鱼类适应静水或缓流环境有关[35, 42],但这一特化具体有何种适应意义尚不清楚. 在本研究的6种高原鳅中,玫瑰高原鳅的听觉阈值位于中等水平(图 3),提示该种鱼鳔的特化对听觉的作用不明显. 当然,膨大的鳔可能接触侧线,鳔的振动更容易刺激侧线神经丘[60],可能有利于对低频振动的感觉.
-
综上,本研究的6种高原鳅中,洞穴鱼类的听觉阈值与非洞穴鱼类相比差异无统计学意义,洞穴鱼类的听觉并没有发生适应性增强,听觉可能对洞穴鱼类适应洞穴环境无明显影响. 种间听觉阈值与鳔室体积和鳔耳距不相关,但在某些频率下,与骨质鳔囊的侧孔面积显著负相关,提示鳔对高原鳅听觉灵敏性的作用主要受侧孔大小的影响. 此外,玫瑰高原鳅鳔的膨大并没有增强其听觉的灵敏性,该种鱼鳔的特化具有何种适应意义还有待研究. 洞穴鱼类的其他非视觉感觉(嗅觉和侧线机械感觉)对适应黑暗的洞穴环境可能更为重要,值得今后的研究关注.






 DownLoad:
DownLoad: