-
开放科学(资源服务)标识码(OSID):

-
痛风性关节炎(Gout Arthritis,GA)是一种由尿酸钠晶体(Monosodium Urate Crystals,MSU)在滑膜、骨、关节囊、软骨和组织中积聚引起的炎症性关节疾病[1-2]。迄今,临床上常用抗痛风急性发作的药物有较大毒副作用,因而寻找无毒副作用或毒副作用小的天然产物备受关注。黑骨藤(Periploca forrestii Schltr,PFS)又叫狭叶蓬莱葛,是中国西南地区的传统药用植物,其提取物具有抗炎、镇痛及强心等功效[3-4]。研究发现,巨噬细胞是参与GA发展的重要效应细胞[5],其极化状态与痛风的发生和发展密切相关[6-7]。黑骨藤醇提取物能否调控巨噬细胞极化状态减轻GA症状与机制还未见报道,本研究通过构建小鼠急性痛风性关节炎模型,观察黑骨藤醇提取物对小鼠痛风性关节炎的防治效果及调控巨噬细胞极化状态的机制。
HTML
-
6~8周龄、体质量20±0.23 g的雄性SPF级C57/BL小鼠共24只,购于川北医学院动物实验中心,在温度22~25 ℃条件下饲养,充足饮食和饮水,每12 h明暗循环。研究严格按照川北医学院动物伦理委员会的方案(2023100)实施。小鼠RAW264.7巨噬细胞购于上海一研生物科技有限公司。
-
秋水仙碱片,云南西双版纳药业有限责任公司;尿酸钠晶体,美国Sigma公司;IL-1β、TNF-α、IL-4、IL-6、IL-10试剂盒,上海茁彩生物科技有限公司;BCA蛋白定量试剂盒,碧云天生物技术研究所;诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)和精氨酸酶-1(arginase-1,Arg-1)抗体,Proteintech公司;低氧诱导因子-1α(hypoxia inducible factor-1α,HIF-1α)抗体,Abclonal公司;磷酸化腺苷酸活化蛋白激酶(phosphorylated AMP-activated protein kinase,p-AMPK),Affbiotech公司;AMPK抑制剂Compound C,北京德航五洲科技有限公司。
-
黑骨藤(PFS)药材采集于贵州省贵阳市,由川北医学院药学院分离提取与鉴定[8]。取黑骨藤药材120 g粉碎,加入70%乙醇1 200 L,在70 ℃水浴中回流提取2 h,过滤,重复3次,合并滤液;分别用石油醚和正丁醇萃取,正丁醇溶剂在真空蒸发器中蒸发,生成正丁醇组分;将最终提取液干燥即得到PFS醇提取物活性成分,最后将混合物配制成1 g/mL的药汁并灭菌包装。
-
随机将24只C57/BL雄性小鼠分为4组,对照组和模型组给予7 d生理盐水灌胃;秋水仙碱组与黑骨藤醇提取物组每天分别用秋水仙碱0.3 mg/kg或黑骨藤醇提取物50 mg/kg连续灌胃7 d;第5 d给药时,除对照组外其余3组在小鼠右踝关节腔注射25 mg/mL的尿酸钠混悬液50 μL,构建急性痛风性关节炎模型[9]。造模前及造模后48 h测定小鼠右踝关节足趾容积并进行步态评分,肿胀度(S)的计算公式[10]为:
式中:V测定为测定时间点足容积;V初始为初始足容积。
步态评分标准[9]:步态正常,计0分。静止时,双后肢着地无明显差异;活动时,患侧肢体承重减轻,轻度跛行,计1分。不管静止还是活动状态下,患侧肢体承重均明显减弱,明显跛行,计2分。患侧肢体不能承重,完全离地,三足步态,计3分。
-
第7 d灌胃1 h后,颈椎脱臼处死小鼠。取小鼠建模侧踝关节,4%多聚甲醛固定,EDTA脱钙60 d,石蜡包埋并切片,苏木精—伊红(hematoxylin-eosin staining,HE)染色,显微镜观察,采用数字病理切片扫描进行拍片。
-
末次给药1 h后心脏穿刺采血,静置2 h,3 000 r/min离心20 min,取上清液,ELISA法检测小鼠血清中IL-1β、IL-6、TNF-α、IL-4、IL-10水平。
-
一组切片脱蜡脱水,柠檬酸钠抗原修复,使用CD68、iNOS、Arg1作为一抗,染色完成后用荧光倒置显微镜检测分析;另一组切片脱蜡脱水,微波修复抗原,加p-AMPK和HIF-1α后4 ℃过夜,DAB显色,苏木精复染,中性树胶封片,并采用Image J软件分析其阳性表达的吸光度值,计算阳性率。
-
将1×105个/孔的RAW 264.7巨噬细胞接种于6孔板,每组做3个复孔。将细胞分为对照组(常规培养)、尿酸钠组(MSU组,100 μg/mL MSU混悬液刺激6 h)、尿酸钠+黑骨藤组(MSU+PFS组,在MSU组基础上加入50 μg/mL的PFS,处理12 h)、尿酸钠+黑骨藤+Compound C组(MSU+PFS+CC组),在MSU+PFS组基础上加入5 μmol/L的Compound C处理2 h)。
-
将RAW264.7巨噬细胞接种于96孔板中,5×104个/孔,分别设置空白组、对照组和给药组。将孔板放置在条件为37 ℃、5%CO2的培养箱中培养24 h,之后将培养基更换为含有不同药物浓度(0、25、50、100、200、400 μg/mL)的黑骨藤培养基,每孔加入100 μL,并继续孵育12 h。在检测前,每孔避光加20 μL MTT(5.0 mg/mL)培养4 h,遗弃培养液,每孔加150 μL DMSO,37 ℃孵育10 min,570 nm处测定OD值并计算细胞活力。
-
收集各组巨噬细胞,用RIPA裂解液从细胞中提取总蛋白。使用等量蛋白质上样,选择10%的十二烷基硫酸钠—聚丙烯酰胺凝胶电泳(SDS-PAGE)进行分离;然后全部转移至聚偏氟乙烯(PVDF)膜上,脱脂奶粉封闭1 h,加入一抗iNOS、Arg1、p-AMPK、HIF-1α,TBST冲洗,后加入二抗,ECL暗室显色,Image-Pro Plus分析蛋白表达水平。
-
收集各组巨噬细胞及培养液,使用ATP含量试剂盒检测各组RAW264.7巨噬细胞中ATP的含量;吸取上清液,使用葡萄糖和乳酸含量检测试剂盒分别检测葡萄糖消耗水平和乳酸分泌水平,具体步骤参照说明书。
-
采用SPSS 26.0统计学软件进行数据处理,结果以x±s表示;采用单因素方差分析,p<0.05为差异有统计学意义。
1.1. 实验动物和细胞
1.2. 主要药品与试剂
1.3. 黑骨藤醇提取物的制备
1.4. 急性痛风性关节炎的建模与关节肿胀评价
1.5. 小鼠踝关节病理形态学观察
1.6. ELISA测定血清TNF-α、IL-1β、IL-6、IL-10、IL-4的分泌情况
1.7. 免疫学方法检测iNOS、Arg1、p-AMPK和HIF-1α的蛋白表达
1.8. RAW 264.7巨噬细胞模型的构建及给药
1.9. 采用MTT法检测RAW 264.7巨噬细胞活力
1.10. 免疫印迹法检测iNOS、Arg1、p-AMPK、HIF-1α的蛋白表达水平
1.11. RAW 264.7巨噬细胞内ATP、葡萄糖摄取量、乳酸生成量的测定
1.12. 统计学分析
-
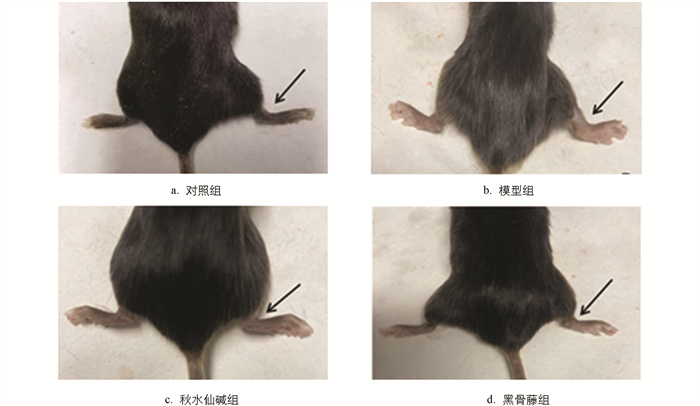
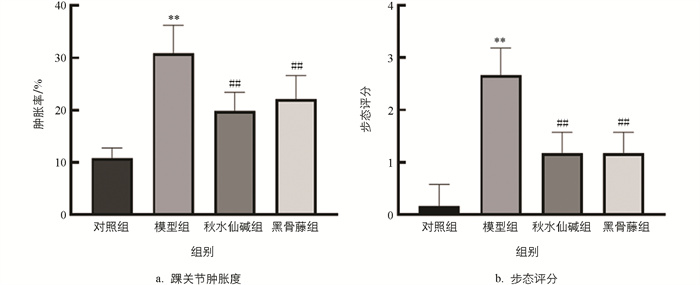
小鼠踝关节肿胀程度见图 1,结果显示,与对照组比较,模型组踝关节肿胀度和步态评分均显著升高;与模型组比较,秋水仙碱组和黑骨藤组踝关节肿胀度和步态评分均显著降低(图 2)。
-
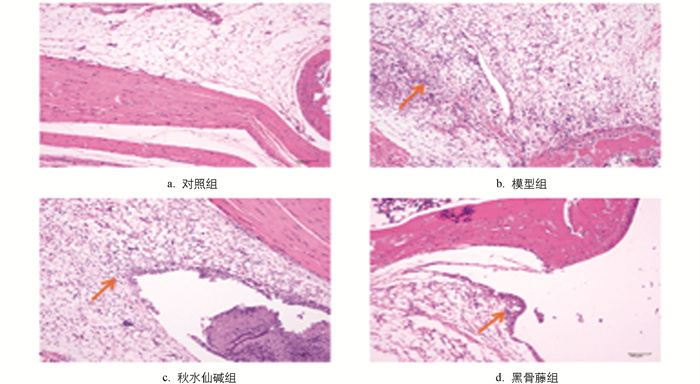
由图 3可知,对照组小鼠踝关节滑膜细胞排列整齐,周围软组织未出现炎细胞浸润;模型组小鼠踝关节中,滑膜细胞增生明显,可见大量炎细胞浸润、纤维素样物质渗出、水肿、出血和新生毛细血管形成;秋水仙碱组小鼠踝关节中滑膜细胞基本保持正常状态,仅有少量炎细胞浸润;黑骨藤组小鼠关节中,滑膜细胞略有增生,充血水肿不明显,可见少量炎细胞浸润。
-
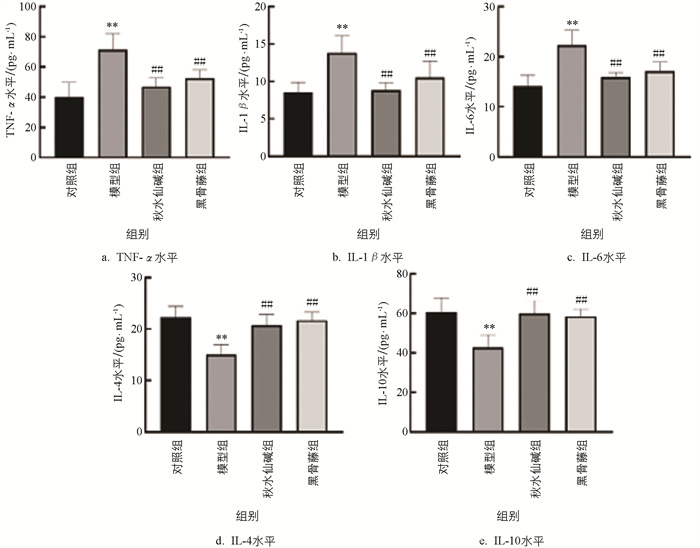
由图 4可知,模型组小鼠血清TNF-α、IL-1β和IL-6水平显著升高,IL-4、IL-10水平显著降低;与模型组比较,秋水仙碱和黑骨藤组小鼠血清中TNF-α、IL-1β、IL-6水平显著降低,IL-4和IL-10水平显著升高。
-
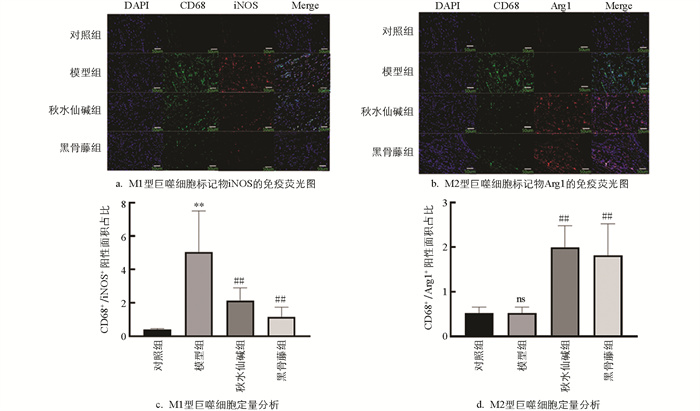
绿色染料标定CD68,红色染料标定iNOS和Arg1,蓝色染料标定细胞核,通过这种方式可以对活化的M1和M2巨噬细胞进行区分。M1巨噬细胞在膜表面高表达CD68和iNOS,而M2巨噬细胞则高表达CD68和Arg1[11]。由图 5可知,模型组小鼠关节滑膜组织中M1细胞的标记物iNOS蛋白荧光强度显著增强;与模型组比较,黑骨藤组和秋水仙碱组小鼠关节滑膜组织中iNOS蛋白荧光强度明显下降,而M2细胞的标记物Arg1蛋白荧光强度显著增强。
-
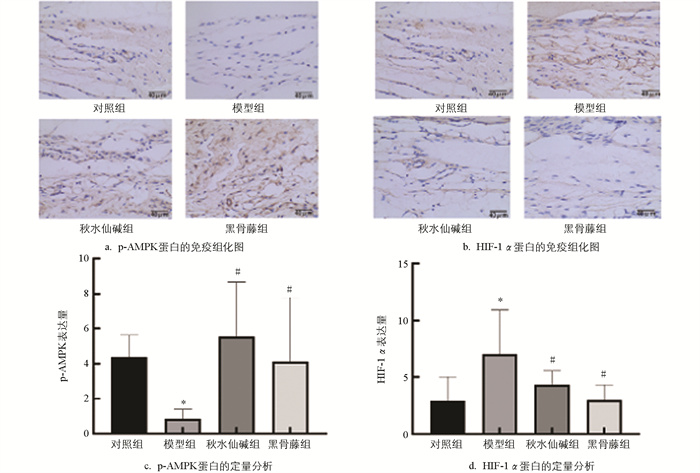
由图 6可知,模型组小鼠滑膜组织p-AMPK蛋白水平明显降低,HIF-1α蛋白水平明显升高;与模型组比较,秋水仙碱组和黑骨藤组p-AMPK蛋白水平明显升高,HIF-1α蛋白水平明显降低。
-
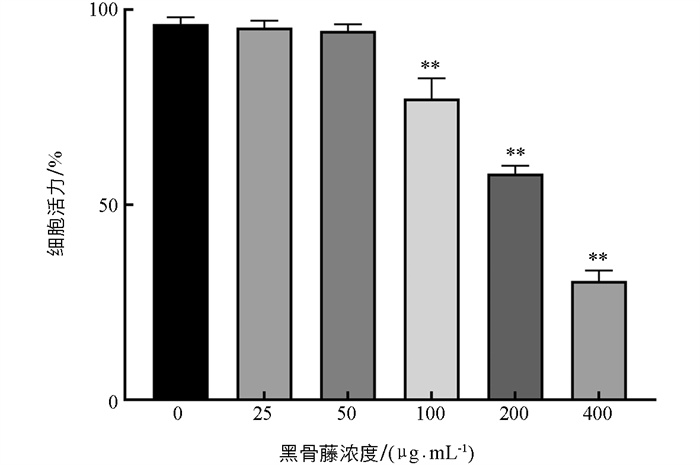
由图 7可知,当黑骨藤浓度大于50 μg/mL时,对细胞活力有显著抑制作用;25、50 μg/mL的黑骨藤对细胞活性影响不大,提示在该浓度范围内对细胞没有明显毒性作用,故本实验选用高浓度50 μg/mL的黑骨藤进行后续的细胞实验。
-
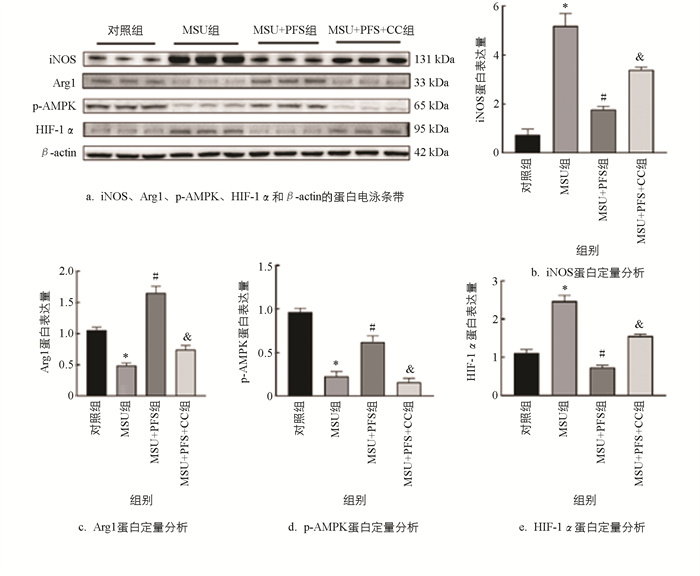
由图 8可知,尿酸钠组(MSU组)iNOS和HIF-1α蛋白水平明显增加,Arg1和p-AMPK蛋白水平显著降低;与MSU组比较,尿酸钠+黑骨藤组(MSU+PFS组)iNOS和HIF-1α蛋白水平明显降低,Arg1和p-AMPK蛋白水平显著增加;与MSU+PFS组比较,尿酸钠+黑骨藤+Compound C组(MSU+PFS+CC组)iNOS和HIF-1α蛋白水平明显增加,Arg1和p-AMPK蛋白水平显著降低。
-
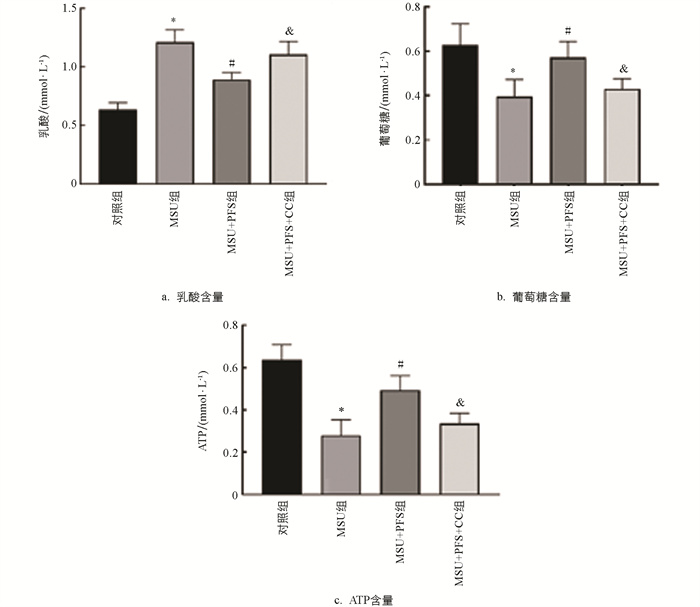
由图 9可知,MSU组细胞内ATP水平降低,乳酸排泄和葡萄糖消耗增加;与MSU组比较,MSU+PFS组巨噬细胞内ATP水平增加,乳酸排泄和葡萄糖消耗降低;与MSU+PFS组比较,MSU+PFS+CC组细胞内ATP水平明显降低,乳酸排泄和葡萄糖消耗明显增加。
2.1. 各组小鼠踝关节肿胀度与步态评分
2.2. 小鼠踝关节组织病理学观察
2.3. 各组小鼠血清炎症因子水平检测
2.4. 各组小鼠踝关节组织巨噬细胞极化标志物iNOS和Arg1的表达情况
2.5. 各组小鼠滑膜组织p-AMPK、HIF-1α蛋白的表达情况
2.6. 不同浓度黑骨藤对RAW264.7巨噬细胞活性的影响
2.7. 黑骨藤通过AMPK/HIF-1α通路对巨噬细胞极化的影响
2.8. 黑骨藤对尿酸钠刺激的RAW264.7巨噬细胞的糖酵解活性影响
-
痛风是临床常见的炎症性关节炎,且近年来其发病率及致残率呈现出逐渐升高的趋势[12]。目前治疗痛风的药物有秋水仙碱、非甾体抗炎药和糖皮质激素,但以上药物均存在一定的副作用,比如胃肠道不适、消化道出血、过敏等[13-14],因此,临床上急需寻找一种安全高效的药物。
黑骨藤(PFS)为西南地区常用药,常用于治疗风湿性、类风湿性关节炎[4, 15-16]。近年来有研究发现,黑骨藤在痛风的治疗中具有显著效果。党荣敏等[17]研究表明黑骨藤醇提物可下调痛风模型大鼠关节的IL-1β、IL-6、IL-8与TNF-α的表达,减轻大鼠踝关节的肿胀,减少滑膜组织炎症细胞的浸润。这一研究结果证实了PFS可有效治疗痛风性关节炎(GA),但其药理机制至今尚未完全阐明,因此本课题组针对黑骨藤治疗痛风性关节炎的机制展开了进一步研究。
巨噬细胞主导的先天免疫是早期GA发生和进展的主要原因[18],也是其发生和进展的重要效应细胞,在不同的微环境中表现出高度的可塑性和异质性,具有对感染和损伤做出快速反应、有利于损伤组织修复的两大关键作用,即巨噬细胞首先极化为促炎表型(M1),然后转变为抗炎表型(M2),以便在直接危险过去时促进修复[19]。MSU晶体在关节腔内聚集并与单核/巨噬细胞系统相互作用是诱导急性痛风性关节炎的关键。在GA小鼠关节组织中,M1型巨噬细胞数量增加,可引起IL-1β、TNF-α和IL-6等促炎细胞因子的过量产生,大量的促炎细胞因子会导致免疫失衡,促进炎症的发展。M2型巨噬细胞能够分泌IL-4和IL-10等抗炎细胞因子,可缓解关节炎症并促进组织损伤修复,因此,在痛风的炎症发展中,巨噬细胞从M1到M2的极化可能有助于急性炎症的自限性,而极化障碍则导致急性炎症慢性化[20]。M1巨噬细胞高表达iNOS,M2巨噬细胞高表达Arg1,这些标志物的检测已被用于测定巨噬细胞的极化状态[21-25]。在本研究中,PFS给药后能够抑制MSU诱导的GA小鼠M1型巨噬细胞极化及炎症细胞因子IL-1β、TNF-α和IL-6的表达,同时促进M2型极化及抗炎细胞因子IL-4和IL-10的表达。这一结果表明PFS可能通过调节巨噬细胞极化来缓解GA小鼠的炎症反应。
AMPK是一种在进化过程中高度保守的丝氨酸/苏氨酸蛋白激酶,被认为是巨噬细胞极化的调节因子[26]。根据最新的研究,激活AMPK可以加速NF-κB的降解,增加Akt的活性,从而抑制炎症反应,促进M1型巨噬细胞向M2型巨噬细胞的转化[27]。HIF-1α是诱导低氧基因及细胞氧内环境修复的核心调节因子,可调控细胞的生长、增殖、迁移、炎症和凋亡等过程[28-29]。有报道认为HIF-1α是控制炎症细胞的代谢开关,可促进细胞的缺氧适应 [30-31]。激活HIF-1α可以抑制M1型巨噬细胞极化和糖酵解,控制关节和皮肤的炎症反应[32]。M1巨噬细胞主要依靠有氧糖酵解快速提供ATP来合成炎症小体[33]。炎症发生时,活化的M1巨噬细胞中三羧酸循环中间产物琥珀酸产量整体增加[34-35],琥珀酸的增加有助于HIF-1α的稳定,这是巨噬细胞持续产生IL-1β所必需的条件[36]。下调HIF-1α表达,可以抑制MSU诱导的巨噬细胞炎症反应[37]。相反,过表达HIF-1α会导致MSU诱导的巨噬细胞线粒体氧化受损,细胞代谢转向糖酵解代谢途径[38]。本实验监测了巨噬细胞的葡萄糖摄取和乳酸分泌能力以及细胞内ATP水平,结果显示,在MSU诱导的巨噬细胞中,乳酸排泄和葡萄糖消耗水平升高,细胞内ATP水平降低;PFS激活了AMPK途径,抑制HIF-1α过表达,导致有氧糖酵解减少,逆转了M1巨噬细胞的这种代谢。故PFS可能通过HIF-1α途径调节细胞的能量代谢,抑制炎性因子的产生。在本实验中,我们发现PFS能上调p-AMPK、Arg1的表达,下调iNOS、HIF-1α的表达,表明PFS能促进AMPK磷酸化和M2型巨噬细胞极化,减少促炎细胞因子的释放,增加抗炎细胞因子的表达,进而促进急性痛风性关节炎的炎性反应缓解。在MSU诱导的巨噬细胞被施与AMPK抑制剂后,PFS增加了iNOS水平,降低了Arg1水平等,因此,本实验研究结果证实PFS可通过AMPK/HIF-1α途径调节巨噬细胞M1/M2极化以发挥抗炎作用。
综上所述,PFS通过AMPK/HIF-1α来抑制MSU晶体在体内外诱导的炎性反应,并且促进巨噬细胞从M1促炎表型转换为M2抗炎表型,减少炎性细胞因子的释放,增加抗炎细胞因子的释放,最终缓解小鼠急性痛风性关节炎。以上实验结果为中医临床领域运用PFS治疗痛风性关节炎提供了新的理论和实验依据。






 DownLoad:
DownLoad: