-
开放科学(资源服务)标识码(OSID):

-
当前,全球气候变化加剧导致极端温度事件频繁发生,给变温动物的生存带来了前所未有的挑战[1-2]。作为影响变温动物生理和行为(如运动、摄食、生长、免疫等)的关键非生物因子,温度对其生存至关重要[2-3]。然而,变温动物对环境温度的耐受力具有一定限度,长期暴露于高温环境可能导致生理损伤甚至死亡[4-5]。在适宜的温度范围内,变温动物可以通过短期热驯化建立热生理补偿机制,以适应热环境[6],这种机制在不同物种之间存在差异[7-9]。例如,一些物种在低温驯化下具备较大的热耐受范围[10-11],而另一些种类则在中等温度驯化下具有较高的热耐受能力[12-13]。对于无尾两栖动物而言,由于其生活史从水生到半陆生或陆生的转变,需要适应较广的温度变化范围,因此其热耐受能力对种群生存尤为重要[14-15]。
温度是调节宿主微生物群落丰富度的关键因素之一[16-18]。研究表明,宿主微生物群落对环境变化具有显著响应[19-20],温度变化可直接或间接影响微生物的生长与代谢速率,从而改变宿主微生物群落的结构和功能[10, 21-22]。例如,口腔和肠道微生物在宿主的消化、免疫和代谢过程中发挥重要作用,并通过协同作用提高宿主对环境变化的适应能力[23-25]。尽管已有研究关注两栖动物肠道微生物对温度变化的响应[26-27],但口腔微生物对温度变化的响应尚未得到充分研究。
黑眶蟾蜍(Duttaphrynus melanostictus)属于两栖纲(Amphibia)、无尾目(Anura)、蟾蜍科(Bufonidae)和头棱蟾属(Duttaphrynus)[28]。该物种主要分布在中国南方地区,尤其在四川、云南和广西较为常见,攀枝花市也有较多分布。黑眶蟾蜍的繁殖期为5-10月,主要以植物害虫为食,在维护自然生态平衡方面发挥重要作用[29]。由于行动缓慢,该物种较易受环境变化的影响[28]。此外,黑眶蟾蜍的蟾酥、蟾衣等部位具有较高的药用价值[30]。当前关于该物种的研究主要集中于盐度、酸碱度、重金属等环境因子对蝌蚪的适应性影响以及其药用价值[29, 31-34],而成体黑眶蟾蜍的热耐受性及口腔菌群对温度变化的响应鲜见报道。本研究基于2种恒定温度(低温:19 ℃;高温:31 ℃)探讨黑眶蟾蜍的热耐受性和口腔菌群变化,目标为:①评估温度驯化对黑眶蟾蜍热耐受性的影响,明确临界高温(CTmax)和临界低温(CTmin)在不同温度条件下的变化趋势;②研究口腔菌群在不同驯化温度下的多样性和结构变化,探讨微生物群落对温度适应的潜在作用。本研究为全球气候变化背景下无尾两栖类的种群分布及生物多样性保护提供了理论依据。
HTML
-
本实验以黑眶蟾蜍成体作为研究对象。2024年6月底,在攀枝花学院校园内的一个持久性池塘中采集了24只成体黑眶蟾蜍,并将其送至学院两栖爬行动物实验室。实验个体被随机分配至3个塑料箱中(尺寸:58 cm×42 cm×33 cm),每箱8只。个体在实验室环境中适应3 d,期间提供充足的食物,饵料为面包虫(Tenebrio molitor)。
-
适应期结束后,使用皮尺(精度±0.1 cm)测量黑眶蟾蜍的体长(从吻端到体后端的长度),并用电子秤(精度±0.01 g)称取质量。初始平均体长为(6.70±0.12)cm,平均质量为(24.80±1.37)g。随后,将个体随机分为2组(19 ℃组,N=12;31 ℃组,N=12)。每组进一步细分为6个小组(每组2只,19 ℃组和31 ℃组各6组)。将每只个体放入已标记的塑料饲养盒中(尺寸:30 cm×23 cm×10 cm),盒底加入暴晒过的自来水,水深约1 cm,以保持适宜湿度。31 ℃组放置在温控PVC恒温内,温度保持在(31.0±0.5) ℃;19 ℃组置于空调控制的实验室中,温度设定为(19.0±0.5) ℃。驯化期间,每隔1 d投喂面包虫,并更换水,投喂时间固定为早上9:00,光照周期为12L∶12D。驯化持续4周,每周测量并记录动物的体长和质量。为确保分组的均衡性,实验开始前对2组的初始体长和质量进行了统计分析。根据Mann-Whitney检验结果,2组体长和质量无显著差异(质量:W=45.5,p=0.790 2;体长:W=34,p=0.252 3),说明随机分组有效平衡了个体大小的影响。
-
驯化期结束后,选取生命体征正常的黑眶蟾蜍个体(N=20,19 ℃组11只,31 ℃组9只),采用临界温度法(CTmax和CTmin)测定其热耐受性。在实验前,动物禁食24 h,并测量质量和体长。临界高温(CTmax)通过数显恒温水浴锅(型号:HH-600,中国)测定。实验开始时,水温设定为20 ℃,并以0.95 ℃/min的速率升温。水浴锅用隔网分为左右两部分,每部分容纳2只个体同时测试。临界低温(CTmin)在连接外部冷水机(型号:AL-500,中国)的鱼缸(尺寸:50 cm×26 cm×30 cm)中测定。实验开始时水温设定为20 ℃,并以0.28 ℃/min的速率降温,同样使用隔网分隔鱼缸,容纳2只个体。实验过程中,使用水银温度计实时监控水温。每隔1 min,实验员用玻璃棒翻转动物,观察其反应,直到动物失去翻正反应。此时的水温即为CTmax或CTmin。测定结束后,立即将动物放入常温水中恢复。完成CTmax测定后,各组动物返回原驯化环境继续驯化1周,再进行CTmin测定。实验过程中,使用数字温度计(精度±0.1 ℃)实时监测水温变化。
-
在热耐受性测定结束后,将动物放回相应的驯化环境恢复1周。随后,每组随机选取5只动物进行口腔微生物采样。取样前,测量并记录每只动物的质量和体长。所有取样操作均在无菌操作台上进行,实验员全程佩戴医用口罩和一次性乳胶手套。正式采样前,使用75%酒精对周围环境及取样人员进行全面消毒。采样时,使用一次性无菌拭子轻轻拭取动物的口腔黏液,拭子随即放入含75%酒精的2 mL无菌离心管中,并迅速存入-80 ℃冰箱。样品通过液氮罐送至天津极智基因科技有限公司进行16S rRNA测序。
采用CTAB法提取口腔微生物总DNA,并通过1%琼脂糖凝胶电泳检测DNA样品的纯度与浓度。凝胶以1×TAE缓冲液制备,添加GelRed染色剂。将5 μL DNA样本与1 μL 6×上样缓冲液混合后上样,电泳条件设定为120 V,30 min。使用Bio-Rad Gel Doc XR+成像系统观察条带完整性,并与λ-HindIII DNA Marker对比,确保样品无明显降解。随后,采用Nano Drop 2000(Thermo Fisher Scientific)测定A260/A280比值,确保所有样本比值在1.8~2.0,排除蛋白或其他污染物的影响,并使用Qubit 3.0荧光定量仪测定DNA浓度,确保符合测序要求。接着,以515F(5'-GTGCCAGCMGCCGCGGTAA-3')和907R(5'-CCGTCAATTCCTTTGAGTTT-3')为引物,使用New England Biolabs公司Phusion® High-Fidelity PCR Master Mix with GC Buffer及高效高保真酶对细菌16S rRNA V4-V5区进行PCR扩增。PCR反应条件如下:98 ℃预变性1 min;98 ℃变性10 s,50 ℃退火30 s,72 ℃延伸30 s,共进行30个循环;72 ℃终延伸5 min。使用2%琼脂糖凝胶电泳检测PCR产物的特异性和完整性,并对样品进行定量,以确保文库均一性。PCR产物检测后,按等量混合,使用NEBNext® UltraTM Ⅱ DNA Library Prep Kit构建文库。文库构建前,通过Qubit和Q-PCR检测文库浓度,确保足够的DNA量进入Illumina NovaSeq 6000平台进行PE 250测序。测序后,对原始数据进行质量控制,包括去除低质量序列、接头序列和嵌合序列(Chimeric Reads),以保证数据的可靠性。所有测序工作由天津极智基因科技有限公司完成。
-
驯化反应率(Acclimation Response Ratios,ARR)是衡量动物驯化能力的关键指标,也是评估机体热适应可塑性的一个重要参数[35-36]。以CTmax为例,ARR的计算公式为:
式中:ΔCTmax为不同驯化条件下CTmax平均值的差值;ΔT为不同驯化温度之间的差值。
统计分析使用Rv 4.4.1软件[37]进行。首先,使用Shapiro-Wilk test检验测试数据正态性,然后采用Scheirer-Ray-Hare检验评估温度对黑眶蟾蜍生长发育的主效应,并通过Spearman相关性分析测定质量、体长与CTmax和CTmin之间的关系。接着,使用Kruskal-Wallis检验分析不同驯化温度下黑眶蟾蜍CTmax和CTmin的差异。最后,计算黑眶蟾蜍的驯化反应率。显著性水平设定为p=0.05,所有描述性统计结果均以平均值±标准误表示。使用ggplot2包和corrplot包对统计结果进行可视化展示。
-
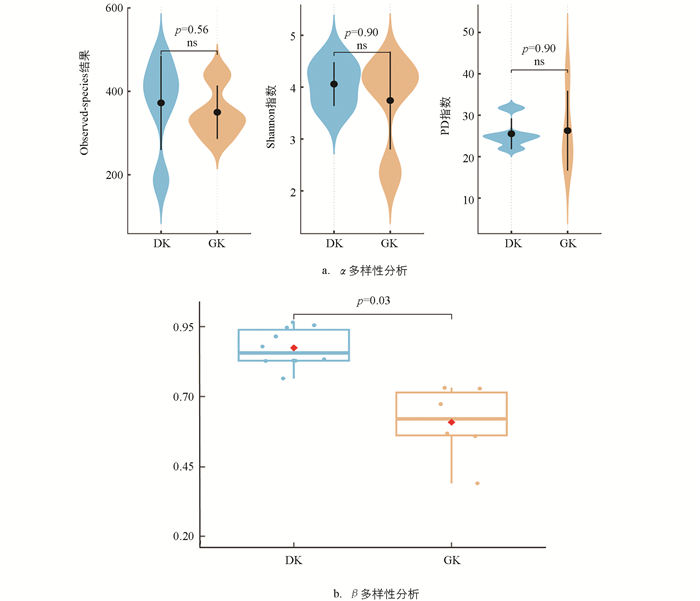
首先,使用QIIME2[38-39]将OTUs代表序列与Silva数据库进行比对,并通过RDP classifier分类器[40]进行物种注释。接着,利用Rv 4.4.1软件中的phyloseq包对数据进行整合和标准化处理。随后,计算α多样性指标,包括Observed-species、Shannon指数和PD指数,并使用dplyr包进行Wilcoxon秩和检验。在β多样性分析中,采用Bray-Curtis距离进行计算,并使用vegan包中的adonis函数进行PERMANOVA检验分析组间差异。然后,利用ape包中的pcoa函数进行主坐标分析(Principal Coordinates Analysis,PCoA)。显著性水平设定为p=0.05,最后,使用ggplot2包对分析结果进行可视化处理。
1.1. 实验材料
1.2. 温度驯化
1.3. 热耐受性测定
1.4. 口腔微生物采样
1.5. 数据分析与处理
1.5.1. 热耐受性数据分析
1.5.2. 口腔微生物数据分析
-
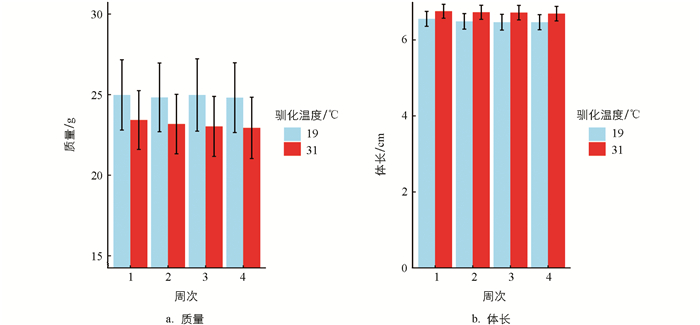
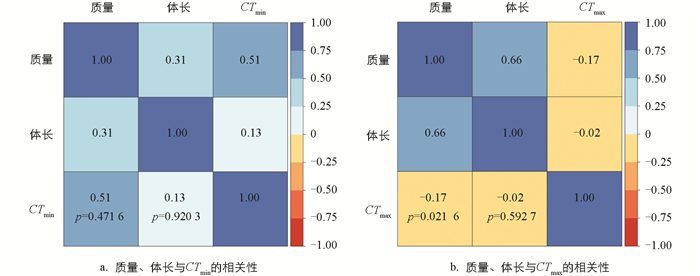
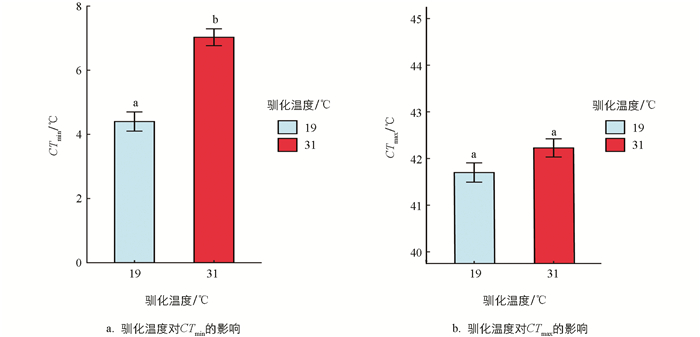
经19 ℃和31 ℃条件下分别驯化4周,结果显示,驯化温度对黑眶蟾蜍的质量和体长未产生显著影响(表 1)。31 ℃驯化条件下,动物个体的质量呈现轻微下降(图 1)。黑眶蟾蜍的CTmax和CTmin与体长无显著相关性,CTmax与质量呈显著正相关,但CTmin与质量无显著相关性(表 1、图 2)。19 ℃组黑眶蟾蜍的CTmin为4.4 ℃;31 ℃组黑眶蟾蜍的CTmin为7.03 ℃,随着驯化温度的上升,CTmin显著提高(x2=13.152 0,p=0.000 3)。19 ℃组黑眶蟾蜍的CTmax值为41.70 ℃,31 ℃组黑眶蟾蜍的CTmax为42.23 ℃,CTmax值不受驯化温度的影响(x2=1.307 5,p=0.252 8)(图 3)。
-
研究结果显示,在19 ℃和31 ℃驯化条件下黑眶蟾蜍CTmin的驯化反应率高于CTmax的驯化反应率。其中,CTmin的驯化反应率为0.26,表明随着驯化温度的升高,黑眶蟾蜍的CTmin增加;CTmax的驯化反应率为0.05,表明驯化温度对黑眶蟾蜍的CTmax影响较小。
-
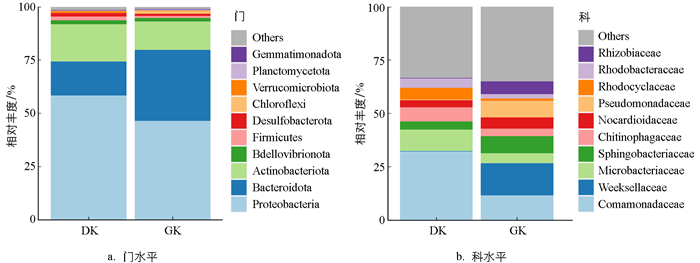
研究结果如图 4所示,不同温度驯化后,黑眶蟾蜍口腔菌群在门和科水平上的组成未表现出明显差异。在门水平上,19 ℃(DK)驯化组和31 ℃驯化组(GK)的黑眶蟾蜍口腔菌群主要优势菌门为Proteobacteria(变形菌门,DK:58.47%、GK:46.58%);Bacteroidota(拟杆菌门,DK:15.98%、GK:33.26%)和Actinobacteriota(放线菌门,DK:17.45%、GK:13.35%)也占比较高。在科水平上,19 ℃驯化组的黑眶蟾蜍口腔菌群中,相对丰度较高的菌科包括Comamonadaceae(丛毛单胞菌科,32.16%)、Microbacteriaceae(微杆菌科,10.08%)、Chitinophagaceae(噬几丁质菌科,6.66%)。相比之下,31 ℃驯化组的黑眶蟾蜍口腔菌群中,相对丰度较高的菌科为Weeksellaceae(威克斯氏菌科,15.13%)、Comamonadaceae(丛毛单胞菌科,11.57%)、Sphingobacteriaceae(鞘氨醇杆菌科,8.06%)、Pseudomonadaceae(假单胞菌科,7.82%)、Rhizobiaceae(根瘤菌科,6.04%)。
-
在α多样性分析中,Observed-species结果显示,19 ℃驯化组(DK)的中位数略高于31 ℃驯化组(GK),但两组比较差异不显著(p=0.56),表明驯化温度对黑眶蟾蜍口腔菌群的物种丰富度影响不大;同样,两组间的Shannon指数未表现出显著性差异(p=0.90),这表明在不同温度驯化条件下,黑眶蟾蜍口腔菌群的物种多样性保持相对稳定;此外,PD指数分析结果也显示两组间无显著差异(p=0.90),表明驯化温度对黑眶蟾蜍口腔菌群的系统发育多样性影响有限(图 5a)。因此,α多样性分析结果表明,不同的驯化温度对黑眶蟾蜍口腔菌群的菌群丰富度、多样性及系统发育多样性均未产生显著影响。在β多样性分析中,基于Bray-Curtis距离的分析结果显示,19 ℃驯化组(DK)和31 ℃驯化组(GK)之间的口腔菌群组成存在显著差异(p=0.03),表明不同驯化温度显著改变了黑眶蟾蜍口腔菌群的群落结构(图 5b)。
-
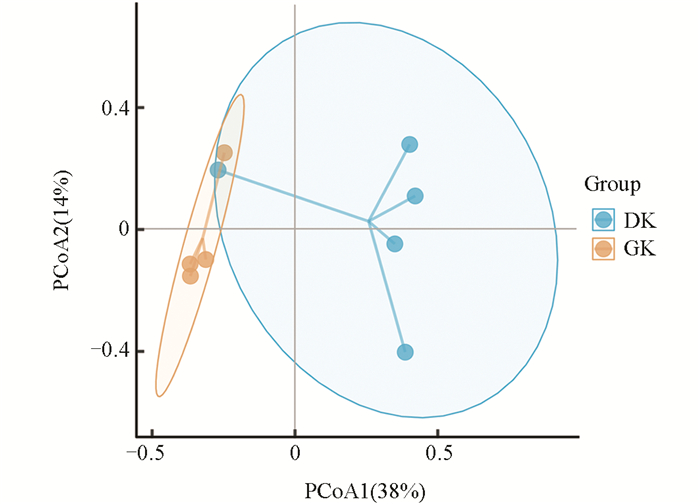
主坐标分析结果显示(图 6),在不同温度驯化条件下,黑眶蟾蜍口腔菌群的群落结构存在显著差异。PCoA1和PCoA2分别解释了口腔菌群结构总变化的38%和14%。19 ℃驯化组(DK)和31 ℃驯化组(GK)分别聚集在不同区域,且两组间的菌群分布相对分离,表明温度驯化对黑眶蟾蜍口腔菌群组成产生了显著影响。这一结果与β多样性分析一致,进一步支持了驯化温度在塑造黑眶蟾蜍口腔菌群结构方面的潜在作用。
2.1. 驯化温度对黑眶蟾蜍热耐受性的影响
2.2. 驯化温度对黑眶蟾蜍驯化反应率的影响
2.3. 不同驯化温度对黑眶蟾蜍口腔菌群的影响
2.3.1. 口腔菌群组成
2.3.2. 不同温度驯化下黑眶蟾蜍口腔菌群多样性分析
2.3.3. 不同温度驯化下黑眶蟾蜍口腔菌群相似度分析
-
在19 ℃和31 ℃条件下驯化一个月后,黑眶蟾蜍的质量和体长未发生显著变化,但31 ℃驯化组的个体质量略有下降。类似结果也在北蝗蛙(Acris crepitans)中观察到,其中25 ℃驯化的个体质量显著低于15 ℃驯化的个体[41]。这种现象符合温度—体型大小规则(Temperature-Size Rule,TSR),即温度升高会导致外温动物体型减小[42],其原因在于温度升高会增加外温动物的能量需求,但短期内食物摄入量可能未能同步增加[43]。因此,在较高温度下,能量摄入和消耗之间的不平衡可能导致黑眶蟾蜍质量的轻微下降。在较高温度下,黑眶蟾蜍体内酶的活性提高,代谢率和运动能力增强,但摄取的食物未能完全满足其代谢需求,导致质量略有下降。类似情况已在其他外温动物中得到证实[44-45]。此外,研究还发现,黑眶蟾蜍的临界耐热温度(CTmax)随着体质量的增加而升高。对于变温动物,不同体型的个体比表面积不同,这会影响其能量效率和热传导能力。因此,质量较大的个体通常具有更强的热耐受能力,这一现象在某些鱼类中已有研究证实[46-47]。
虽然驯化温度对黑眶蟾蜍的临界高温(CTmax)无显著影响,但对临界低温(CTmin)有显著影响,即低温驯化的个体比高温驯化的个体具有更强的低温耐受力,反之则不然。这一现象可能与攀枝花市当地干热河谷气候特点有关。该地区年平均气温较高,夏季温度甚至可达40 ℃[48],黑眶蟾蜍可能早已适应了当地的高温环境,因此驯化温度对其CTmax的影响不明显。相反,温度变化对CTmin的影响较大,尤其在高温驯化环境中,黑眶蟾蜍的耐低温能力显著减弱。类似低温耐受的可塑性在其他外温动物中也有报道,如蟾蜍(Rhinella icterica)[49]、大西洋白姑鱼(Argyrosomus regius)[50]和北美细趾蟾(Eleutherodactylus coqui)[51]。黑眶蟾蜍的低温耐受可塑性可能是其适应干热河谷环境的一种策略,通过温度驯化来应对短期的低温暴露。这种表型可塑性可能有助于其在温度变化的栖息地中生存,但如果出现异常低温天气,黑眶蟾蜍的生存可能会受到威胁。随着全球气候变化,极端天气的频发可能对该物种的生存构成更大的挑战。
ARR反映了外温动物对不同驯化温度变化的生理响应能力。ARR值越大,表明动物生理功能对温度变化的可塑性越强[36, 52]。本研究发现,黑眶蟾蜍CTmin的ARR值(0.26)显著高于CTmax(0.05),表明其热耐受在低温(19 ℃)时的可塑性较高,而在高温(31 ℃)时的可塑性较低。这个结果与其他变温动物的研究结果相似,例如,东方蝾螈(Cynops orientalis)[53]、斑马鱼(Danio rerio)[54]、美洲林蛙(Bufo marinus)[55],但与一些表现出CTmax的ARR值大于CTmin的物种有所不同[7-8, 45]。这种差异反映了不同栖息环境中的动物扩展耐受能力的策略差异,可能与其所处环境的温度波动模式有关。生活在短期温度波动较大环境中的物种,可能比生活在温度长期缓慢变化环境中的物种具有更强的抵抗快速温度变化的能力[56-57]。
-
两栖动物的基本生理功能直接依赖于环境条件[58-59],因此其行为、生理和菌群组成极易受到环境温度的显著影响[4, 26]。近年来,关于两栖动物肠道和皮肤微生物多样性对温度响应的研究逐渐增多[17, 60-62]。然而,关于温度对两栖动物口腔菌群的影响鲜见报道。本研究发现,黑眶蟾蜍在19 ℃和31 ℃驯化条件下的口腔菌群组成在门水平上基本相似,以Proteobacteria(变形菌门)为主导,其次为Bacteroidota(拟杆菌门)和Actinobacteriota(放线菌门),表明驯化温度对黑眶蟾蜍口腔菌群在门水平上的组成影响较小。一方面,Proteobacteria因其广泛的环境适应性和代谢多样性,能够在不同温度下保持主导地位[63]。另一方面,Bacteroidota和Actinobacteriota在有机物分解和免疫调节方面具备一定优势,能够在温度波动中保持稳定性[64-65]。此外,驯化期间食物来源的单一性和稳定性也可能有助于维持口腔菌群的组成稳定性,这一现象在红腹蟾蜍(Melanophryniscus admirabilis)的研究中也得到了验证[66]。在科水平上,不同驯化温度下黑眶蟾蜍口腔菌群的优势菌科存在显著差异。在19 ℃驯化组中,优势菌科包括Comamonadaceae(丛毛单胞菌科)、Microbacteriaceae(微杆菌科)和Chitinophagaceae(噬几丁质菌科)。这些菌科的存在可能与低温环境中两栖动物口腔的生态需求相关。例如,Comamonadaceae在水生环境中具有较强的有机物分解能力,能够帮助分解口腔中的有机物质,减少有害代谢物的积累[67];Microbacteriaceae以分解复杂的多糖类物质等有机物为主,能够清除口腔中的残留食物和碎屑,为宿主提供有益的代谢产物[68-69];Chitinophagaceae以分解几丁质等硬壳多糖为主,不仅可以清理口腔中可能残留的昆虫外骨骼和其他含几丁质的有机物,提供可利用的营养成分,还可能具有保护宿主免受外源致病菌感染的作用[70]。相较之下,31 ℃驯化组的优势菌科包括Weeksellaceae(威克斯氏菌科)和Pseudomonadaceae(假单胞菌科),这可能与这两类菌的高温适应性及其在高温环境下的特殊代谢功能相关。例如,Weeksellaceae和Pseudomonadaceae在高温环境中具有较强的食物残渣和有机质分解能力,有助于减少宿主口腔中的有机残留物,帮助维持口腔内环境的稳态[63, 71],并对病原菌具有一定的抑制作用[72]。
本研究结果表明,尽管19 ℃和31 ℃驯化条件下的黑眶蟾蜍的口腔菌群多样性相似,但群落结构存在显著差异。19 ℃驯化组的口腔菌群结构表现出较高的组内变异性,而31 ℃驯化组的菌群结构较为一致。这可能是环境因素、宿主生理特性及微生物群落动态平衡共同作用的结果。已有研究表明,两栖动物的微生物群落结构常受到环境温度、湿度和水质等因素的显著影响[73]。此外,黑眶蟾蜍口腔菌群的α多样性指标(物种丰富度和均匀度)相似,但宿主免疫反应可能对特定微生物种群的丰度产生影响,进而改变群落结构。相关研究发现,两栖动物在不同发育阶段的微生物群落结构存在显著差异[74]。在不同环境压力或宿主因素的影响下,黑眶蟾蜍口腔菌群的动态平衡可能发生变化,从而导致口腔菌群群落结构的显著差异。宿主微生物群落在压力环境下会发生结构变化,以适应新的生存条件。例如,两栖动物的皮肤微生物在高温或化学物质暴露下会调整群落结构,以增强其适应性[74]。总之,这些结果强调了微生物群落的复杂性和适应性,未来研究可进一步探讨验证这些因素对黑眶蟾蜍及其他两栖动物口腔微生物群落的影响。
-
本研究探讨了驯化温度对黑眶蟾蜍热耐受性及其口腔菌群的影响,揭示了温度在其生理适应和微生物群落结构中的多重作用。首先,驯化温度显著影响了黑眶蟾蜍的CTmin,但对CTmax无显著影响,表明黑眶蟾蜍的热耐受下限较易随环境温度变化而调整,展示出较强的生理适应性。其次,α多样性分析发现,不同温度驯化下黑眶蟾蜍口腔菌群丰富度和多样性无显著变化,但β多样性和PCoA分析揭示了不同温度驯化条件下黑眶蟾蜍口腔菌群结构的显著差异。这些结果表明,温度不仅影响两栖动物的生理适应,还通过改变微生物群落来进一步调控生理功能。随着全球气候变化加剧,温度波动可能对两栖动物的热适应性和微生态系统产生深远影响。不断上升的环境温度可能导致像黑眶蟾蜍等无尾两栖类物种的耐受极限和微生物群落动态发生变化,进而影响其生存能力和生态平衡。因此,本研究不仅为理解温度适应在野外环境中塑造生物多样性提供了新的视角,还为应对气候变化对生物体微生态的潜在影响提供了重要的科学依据。






 DownLoad:
DownLoad: