-
开放科学(资源服务)标识码(OSID):

-
农田土壤中抗生素污染问题十分普遍,涉及到的抗生素种类较多,污染分布面广,且以复合污染为主[1-2]。据统计,2017年全球抗生素药物销售量约为93 309 t,预计到2030年,全球抗生素销售量将达到104 079 t,涨幅超过11%[3]。目前,磺胺类、四环素类抗生素对土壤的污染以及对作物生长的影响的研究较为广泛[4],而作为应用最广泛的喹诺酮类抗生素之一的恩诺沙星对土壤性质和土壤微生物的影响的研究则相对较少[5]。
抗生素的添加影响土壤中微生物的多样性和群落的稳定性。研究发现,在水稻土的稻田轮作体系中,加入环丙沙星和磺胺甲氧嘧啶后,土壤细菌多样性整体呈下降趋势[6],在水稻体系中添加恩诺沙星,随着添加量的增加,土壤细菌和真菌的丰富度及多样性均显著下降[7]。此外,抗生素进入土壤会影响土壤微生物群落的组成。水稻体系中,添加四环素后土壤Xanthobacteraceae门的相对丰度显著增加,在四环素、磺胺甲氧嘧啶、环丙沙星的共同处理下,Proteobacteria门大量富集[6]。文献[8]研究发现,氧氟沙星的添加显著抑制了真菌群落中Kathrablepharidae属的生长。而在温室大棚中,四环素等抗生素的大量残留对土壤真菌和细菌的施用多样性均具有显著抑制作用[4]。同时,抗生素的添加还会影响微生物群落的稳定性,研究表明,在水稻体系中阿维菌素的施用显著降低了土壤微生物群落的复杂性和稳定性[9]。土壤中抗生素的存在和积累不但会改变土壤微生物的群落特征,还会抑制微生物碳源的利用率及酶的活性等[10]。因此,探究抗生素对土壤微生物组成及结构的影响,对于保持土壤健康,发挥土壤功能具有重要意义。
土壤中与磷循环相关的微生物的丰度和群落结构特征是影响土壤中磷素转化的关键。大多数农田土壤中的全磷较为丰富,但是只有少部分可溶性的有效磷可直接被植物吸收利用,大部分以难溶性的无机态和有机态固定于土壤中。通过微生物手段促进土壤中有机磷的矿化和无机磷的溶解,是提高土壤中磷素有效性的重要途径之一。含phoD基因的细菌群落是土壤中矿化有机磷的重要类群。研究发现,水稻体系中含phoD基因的细菌群落的丰度和组成会直接影响土壤中的磷酸酶活性,具体表现为含有高丰度phoD基因的细菌群落的土壤中磷酸酶含量更高,土壤中磷的有效性也更高[11]。含phoD基因的细菌群落的关键物种对土壤磷循环的调控具有重要作用。研究发现,草原土壤中含phoD基因的细菌群落中Dietzia属和Sphingomonas属与碱性磷酸酶活性的相关性有统计学意义,它们通过矿化有机磷来提高土壤中磷的有效性[12]。此外,在高山森林土壤中,含phoD基因的细菌群落中Chrysosporum属和Anabaenam属与碱性磷酸酶的活性的相关性有统计学意义,而与phoD基因的丰度和多样性无关。吡咯喹啉醌是一种辅助因子,参与生成葡萄糖酸,用于难溶性无机磷的溶解,其中pqqC基因编码了吡咯喹啉醌的合成酶,催化了吡咯喹啉醌的产生,是溶解难溶性无机磷的微生物标记基因[13]。研究发现,玉米体系中pqqC基因的丰度与土壤中植株吸磷量和产量的相关性有统计学意义,表明pqqC基因与土壤磷素的转化和吸收密切相关[14]。因此,研究含phoD和pqqC基因的土壤微生物的多样性、群落组成和土壤磷的有效性,对于探究土壤磷循环过程以及增加土壤可利用磷库具有重要意义。然而,目前与抗生素的添加介导的磷循环微生物对磷素转化的影响相关的研究较少,仍需要对其深入探究。
目前,有机肥替代化肥已在农田生态系统中广泛普及[15-18],本研究通过盆栽试验,在有机肥体系中通过添加抗生素的模拟试验,利用Real-Time qPCR高通量测序技术,结合土壤酶的活性测定、BBP磷分级法,探究不同恩诺沙星的添加对土壤微生物中含有机磷矿化(phoD)、无机磷溶解(pqqC)关键基因的微生物的丰度、群落特征的影响,及其对土壤磷素转化机制的影响。
HTML
-
盆栽试验选取了重庆市潼南区紫色土,研磨风干过5 mm筛备用。土壤pH值为7.8,有机碳含量为3.8 g/kg,速效磷含量为15.1 mg/kg,全氮含量为1.2 g/kg。鸡粪有机肥过5 mm筛,添加比例为土壤干重的2%,鸡粪中含磷20.85 g/kg,含钾33.60 g/kg。底肥为尿素(46%),供试磷、钾肥分别为过磷酸钙(12%)和硫酸钾(50%)。
试验设计3个抗生素水平,设置为0 mg/kg(CK),0.5 mg/kg(Low-ENR),5 mg/kg(High-ENR)。配置不同水平抗生素标液,将风干土平铺于塑料布上,用喷雾器喷洒不同水平的抗生素标液,同时不断翻搅,使土壤与抗生素充分混匀。加蒸馏水调节处理后的土壤初始含水量为65%(质量含水量),随后装入培养盆中。试验采用塑料盆钵,内径22 cm,每盆装土4 kg。供试作物为辣椒(品种为辛香8号),育苗后待辣椒长至6叶期进行移栽,挑选长势均匀的辣椒幼苗,辣椒根系去除基质,蒸馏水清洗后移栽于盆钵,每盆3株,每个处理设置4个重复。作物生长期间定期灌溉,使土壤田间持水量保持在55%左右(质量含水量)。
试验开始后分别于第7 d和第30 d采集盆钵内土样。采样土样时,用20 mm的土钻随机采取同一个盆栽中的土壤,然后将土钻收集到的土壤混合均匀视为一个样品。部分土样风干磨碎后用于后续理化性质测定,部分置于-20 ℃保存用于高通量测序及土壤酶活性的测定。
植株样采集时将样本分为根、茎、叶3部分,用去离子水清洗,擦干表面水分,测定样本鲜重,之后放置烘箱121 ℃杀青,60 ℃烘干72 h,称量各部位的生物量。将样本研磨至粉末,采用微波消解ICP-OES测定其磷素含量。
-
土壤pH值采用酸度计法测定,水土质量比为2.5∶1。土壤有机质采用重铬酸钾容量法测定。土壤速效磷采用钼锑抗比色法测定。土壤全氮采用凯氏定氮法测定。通过测定对硝基苯基磷酸盐(p-Nitrophenyl Phosphate,PNPP)的释放量来估算土壤酸性磷酸酶(Acid Phosphatase,ACP)和碱性磷酸酶(Alkaline Phosphatase,ALP)的活性,在420 nm波长下比色测定,酸、碱性磷酸酶活性以μg PNP h/g soil计[19]。
土壤磷素分级采用基于生物有效性的磷分级方法(Biologically-Based Phosphorus,BBP)[20],由4种不同的提取剂来提取,分别为氯化钙提取态磷(CaCl2-P,0.01 mol/L)模拟自由扩散和根基截留的磷、柠檬酸提取态磷(Citrate-P,10 mmol/L)模拟可被有机酸活化和无机酸弱结合的无机磷(包括钙磷、铝磷和铁磷)、酶提取态磷(Enzyme-P,0.02 EU/mL)模拟酶矿化的有机磷、盐酸提取态磷(HCl-P,1 mol/L)模拟难利用的磷库。后续采用孔雀石绿法测定磷的含量。
-
利用FastDNA SPIN Kit试剂盒提取土壤总DNA,取适量样本进行琼脂糖凝胶电泳以检验DNA提取质量。使用NanoDrop2000核酸检测仪(NanoDrop Technologies,Wilmington,DE)测定所提DNA样本的纯度。
-
采用Real-time PCR法检测样本中phoD与pqqC基因的绝对含量。引物使用ALPs-F730(5′-CAGTGGGACGACCACGAGGT-3′)和ALPs-1101(5′-GAGGCCGATCGGCATGTCG-3′)扩增phoD基因,扩增片段大小为371 bp。使用ApqqCF(AACCGCTTCTACTACCAG)和ARpqqCR(GCGAACAGCTCGGTCAG)[21]扩增pqqC基因,扩增片段大小为306 bp。每个PCR反应体系为20 μL,包含10 μL的2X ChamQ SYBR Color qPCR Master Mix、0.8 μL引物F(5 μM)、0.8 μL引物R(5 μM)、0.4 μL的50 X ROX Reference Dye 1、2 μL DNA、6 μL dd H2O。PCR的扩增条件为:95 ℃ 3 min,之后40个循环的反应条件为95 ℃ 5 s、58 ℃ 30 s和72 ℃ 1 min。首先,将PCR扩增产物克隆到pMD18-T载体质粒中,然后将克隆的质粒插入正确的片段,随后提取,用NanoDrop2000分光光度计测定质粒含量。利用公式
计算质粒DNA拷贝数,同时根据质粒的连续稀释(10倍)绘制标准曲线。
-
使用1%琼脂糖凝胶回收PCR产物,采用DNA凝胶提取试剂盒(Axygen Biosciences,Union City,CA,USA)进行纯化,Tris-HCl缓冲液洗脱,2%琼脂糖电泳检测。使用Illumina Miseq平台进行高通量测序,测序得到的原始测序序列使用fastp(v0.19.6)软件根据测序质量对双端测序后读段(Reads)进行质控和过滤质控,同时根据双端Reads之间的重叠(overlap)关系使用FLASH(v1.2.7)软件进行拼接,获得质控拼接之后的优化数据。然后使用序列降噪方法(DADA2、Deblur等)处理优化数据,获得扩增子序列变体(Amplicon Sequence Variant,ASV)的代表序列和丰度信息。采用RDP classifier贝叶斯算法对ASV代表序列进行分类学分析,获得ASV在分类水平的注释信息。原始数据提交到美国国家生物技术信息中心(National Center for Biotechnology Information,NCBI)数据库,序列号为PRJNA1062280。
-
采用单因素方差分析检验不同时期不同恩诺沙星处理下土壤各项理化性质、细菌群落α-多样性间差异的显著性,在p=0.05的水平上利用Duncan法进行比较。所有的统计分析均基于SPSS分析软件(version 20)和R分析软件(4.2.2)。基于ASV丰度矩阵计算细菌群落的α-多样性(香农指数),利用origin呈图,香农指数计算公式为:
式中:Sobs为实际观测到的物种(如ASV)数,ni为第i个物种(如ASV)所含的序列数,N为所有的序列数。
在ASV水平上基于Bray-Curtis距离对细菌群落进行主坐标分析(Principal Coordinate Analysis,PCoA)。去除丰度较低的ASV(丰度小于0.01%)后,基于SparCC法计算微生物之间的相关性,保留r>0.8,p<0.05数据构建微生物共现网络,之后通过Gephi软件可视化,进而计算网络拓扑性质,包括节点数、连接数、节点度、网络密度、聚类系数等。其中网络中节点的大小与节点度成比例呈现。通过R分析软件(4.2.2)进行细菌群落稳定性(cohesion)的计算,cohesion指数可以量化微生物群落的连通性程度[22]。使用linkET包进行Mantel检验和相关性的计算。
1.1. 基质土采集及性质
1.2. 土壤理化因子及磷酸酶测定
1.3. 土壤总DNA提取
1.4. phoD与pqqC功能基因实时荧光定量
1.5. 含phoD与含pqqC功能基因的Illumina Miseq测序
1.6. 数据分析
-
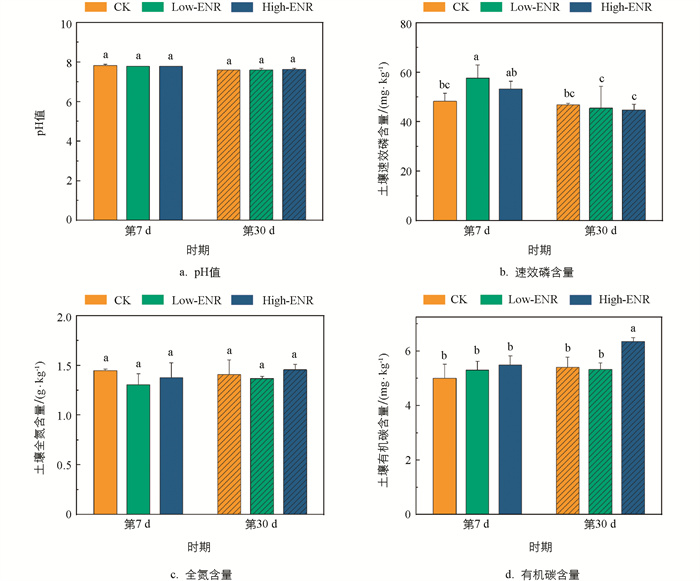
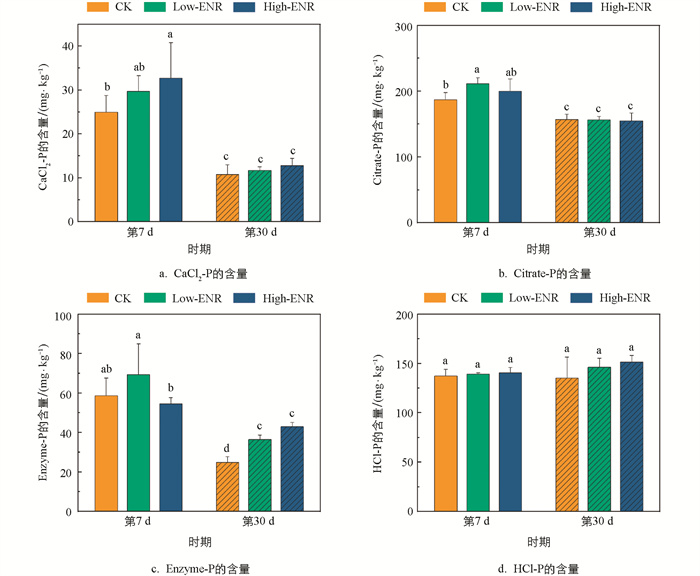
有机肥施用下,恩诺沙星的添加对土壤pH值和全氮的含量在不同时期的影响均无统计学意义(图 1a、c),但分别在第7 d增加了土壤速效磷(Available Phosphorous,AP)的含量,第30 d增加了土壤有机碳(Soil Organic Carbon,SOC)的含量(图 1b、d)。恩诺沙星添加后土壤中CaCl2-P、Citrate-P、Enzyme-P的含量在第7 d显著高于第30 d(p<0.05)(图 2a、b、c),而土壤难利用的HCl-P的含量则在不同时期的相关性无统计学意义(图 2d)。第7 d,土壤中Citrate-P的含量在添加恩诺沙星后显著增加(图 2b);而在第30 d,土壤中Enzyme-P的含量则随着恩诺沙星含量的增加而显著增加(图 2c)。
-
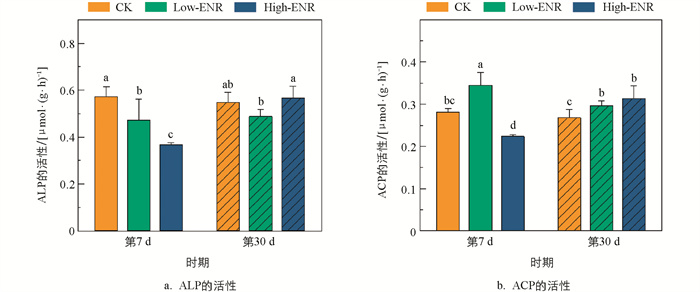
恩诺沙星添加前期(第7 d),土壤中ALP、ACP的活性在High-ENR处理下显著降低(图 3)。值得注意的是,第7 d Low-ENR处理显著促进了土壤ACP的活性。随恩诺沙星处理时间的延长(第30 d),土壤ACP和ALP的活性在不同恩诺沙星处理下的差异无统计学意义。
-
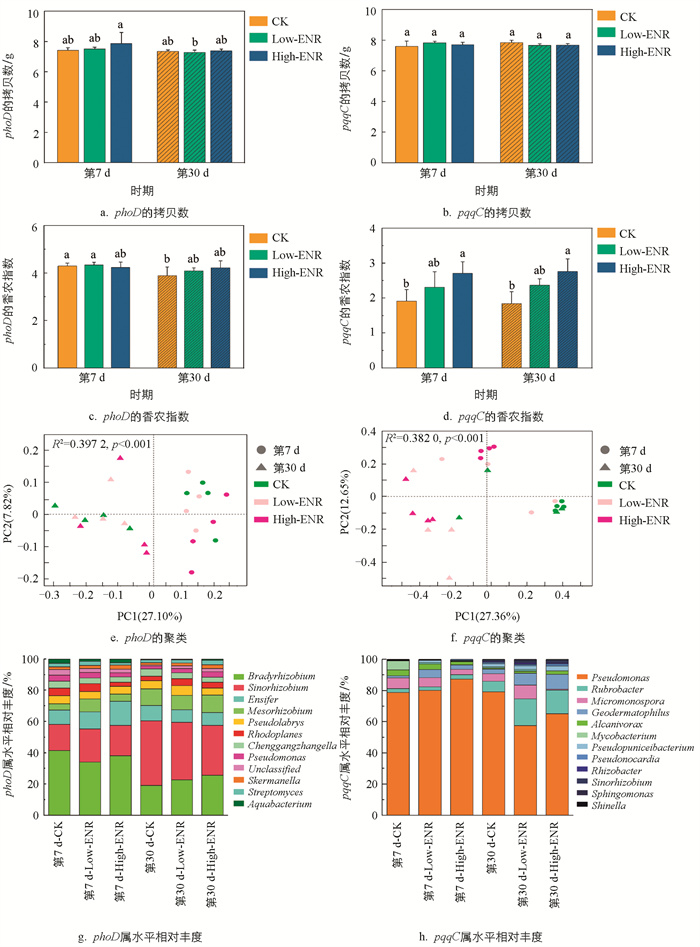
为了探究恩诺沙星的添加对土壤磷循环功能微生物的影响,我们对土壤中含phoD和pqqC基因的细菌群落进行了高通量测序。结果表明,恩诺沙星的添加对土壤中含phoD和pqqC基因的细菌拷贝数影响不大(图 4a、b)。恩诺沙星添加在两个时期均显著影响了土壤中含pqqC基因细菌群落的组成多样性。随着恩诺沙星添加量的增加,pqqC的香浓指数显著增加,这一结果说明恩诺沙星的添加增加了土壤含pqqC基因细菌群落的α-多样性(图 4d)。基于Bray-Curtis距离进行主坐标分析的结果表明,恩诺沙星的添加在两个采样时期均显著影响含phoD和pqqC基因的细菌群落的结构。对于含phoD基因的细菌群落,第7 d或第30 d CK处理和Low-ENR处理沿PC2轴明显聚类,High-ENR处理沿PC2轴单独聚类。而对于含pqqC基因的细菌群落,恩诺沙星添加后,CK处理与High-ENR处理聚类明显(图 4e、f)。另外,不同恩诺沙星处理在不同时期显著影响了土壤中磷循环功能微生物的群落组成。第30 d与第7 d相比,Bradyrhizobium、Sinorhizobium和Pseudomonas的相对丰度显著降低,而Rubrobacter的相对丰度则呈现一定程度的增加(图 4g、h)。
-
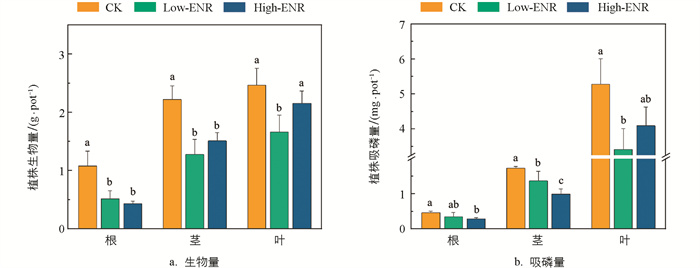
为了探究恩诺沙星的添加对辣椒植株生长和磷吸收的影响,对第30 d辣椒植株各部位的生物量和吸磷量进行了测量。结果发现,恩诺沙星的添加显著降低了辣椒植株各部位的生物量,与吸磷量的结果大体一致。这一结果说明,抗生素恩诺沙星的添加对作物的生长起到了明显的抑制效果,同时抑制了植株对磷的吸收(图 5)。
-
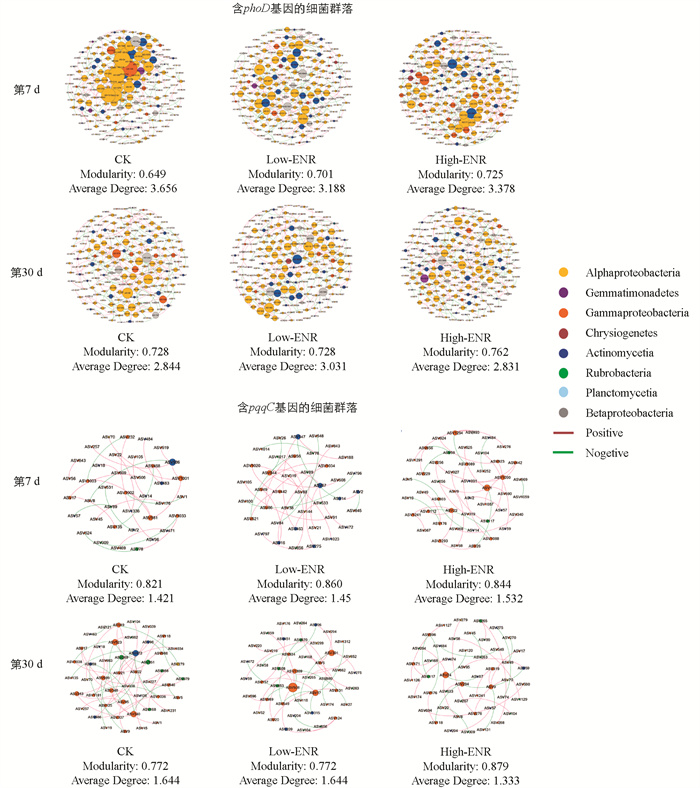
利用SparCC法计算微生物之间的相关性,构建微生物共现网络,计算网络拓扑性质,探究恩诺沙星的添加对含phoD基因、pqqC基因的细菌群落的网络互作功能的影响。结果表明,恩诺沙星的添加影响了含phoD基因、pqqC基因的细菌群落的网络互作(图 6)。恩诺沙星处理显著增加了网络的模块性(Modularity)。值得注意的是,第7 d恩诺沙星的添加降低了含phoD基因细菌群落的网络平均度(Average Degree),而在第30 d则增加了其网络平均度。含pqqC基因的细菌群落的网络平均度的影响与含phoD基因的细菌群落相反。
-
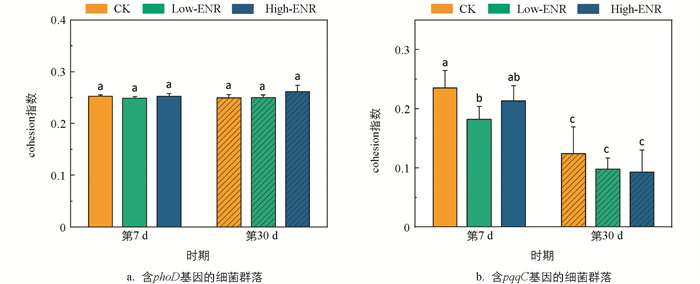
继续探究恩诺沙星的添加对含phoD和pqqC基因的细菌网络的影响。计算其群落的cohesion指数,表征其网络的凝聚度。结果显示,恩诺沙星处理对含phoD基因细菌的cohesion指数并无显著影响。而显著降低了含pqqC基因细菌的cohesion指数(图 7a、b)。说明恩诺沙星的添加会显著降低pqqC基因细菌群落的凝聚度。同时,第30 d与第7 d相比,含pqqC基因细菌群落的cohesion指数显著下降。
-
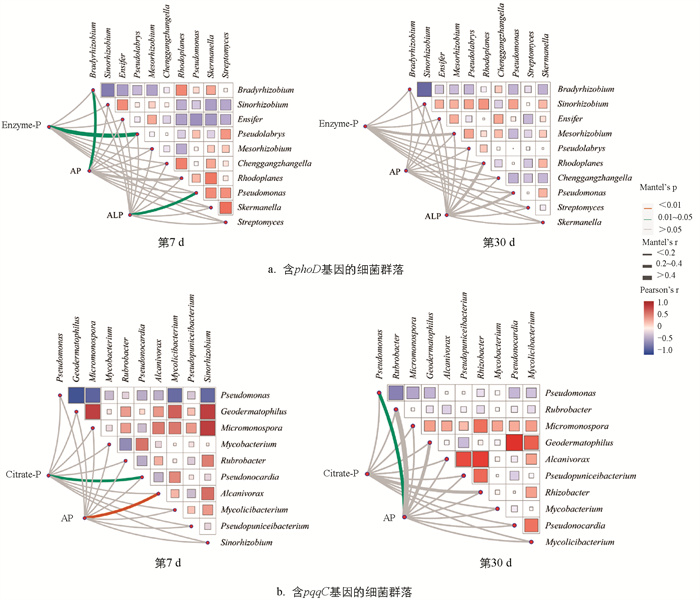
将相对丰度前10的菌属定义为优势属,利用蒙特检验进行磷形态与含phoD、pqqC基因细菌间的相关性分析(图 8)。结果显示,在恩诺沙星添加的第7 d,含phoD基因的优势菌属中,Bradyrhizobium与Pseudolabrys分别与土壤中AP和Enzyme-P的相关性有统计学意义,同时,土壤ALP与Pseudomonas的相关性有统计学意义,到第30 d相关性无统计学意义。含pqqC基因的优势菌属中,第7 d Pseudonocardia与土壤Citrate-P的相关性有统计学意义,Alcanivorax与土壤AP的相关性有统计学意义,第30 d Pseudomonas与土壤AP的相关性有统计学意义。恩诺沙星的添加改变了土壤中磷循环功能微生物与磷组分之间的相关性。
2.1. 有机肥体系恩诺沙星的添加对土壤特性及磷素形态的影响
2.2. 有机肥体系恩诺沙星的添加对土壤碱性磷酸酶和酸性磷酸酶活性的影响
2.3. 有机肥体系恩诺沙星的添加对含phoD和pqqC基因的细菌群落的影响
2.4. 恩诺沙星的添加对辣椒植株生长的影响
2.5. 恩诺沙星的添加对土壤中含phoD、pqqC基因的细菌群落网络互作特征的影响
2.6. 恩诺沙星的添加对含phoD和pqqC基因细菌群落cohesion指数的影响
2.7. 恩诺沙星添加条件下的磷形态与含phoD和pqqC基因的细菌优势属的相关性分析
-
农田生态系统中抗生素往往通过有机肥的施用在土壤中残留、累积[23]。之前的研究发现,添加四环素后土壤中有机磷的含量显著降低[24],而在猪粪改良的水稻土中,有机磷的含量显著增加[25],与本研究结果不同。在本研究中,抗生素的添加整体对CaCl2-P、Enzyme-P以及HCl-P的影响较小,主要在第7 d显著增加了Low-ENR处理下土壤中Citrate-P的含量(图 2)。我们推测,抗生素对土壤有机磷的影响与抗生素的种类及作物体系密切相关。另外,抗生素的施用会对土壤中特定酶的活性产生抑制作用。研究发现,四环素类抗生素施用后,马铃薯—小麦轮作体系土壤中脲酶的活性明显降低[26]。同样,盐酸土霉素、磺胺乙嗪、盐酸环丙沙星、单霉素钠在豌豆体系土壤中抑制了氮同化酶的活性,导致豌豆的氮利用率和产量的下降。磷酸酶是驱动土壤有机磷矿化的关键酶类,土壤酸性磷酸酶与碱性磷酸酶的活性随多粘菌素和青霉素的添加呈低含量促进、高含量抑制的趋势[27]。在本研究中,前期恩诺沙星的添加抑制了土壤碱性磷酸酶的活性,然而酸性磷酸酶的活性显著增加,对土壤中Enzyme-P含量的影响无统计学意义。
Citrate-P代表了土壤中能被有机酸溶解的无机磷。当有机肥施用后,Low-ENR处理在第7 d显著增加了土壤Citrate-P的含量(图 2)。这可能是由于抗生素添加后,会与土壤中的有机酸竞争矿物颗粒上的吸附位点[28],从而导致土壤中参与无机磷活化的有机酸含量增加,导致Citrate-P含量的增加。同时,pqqC基因参与了土壤有机酸的分泌,抗生素添加后影响了土壤含pqqC基因的细菌群落,群落稳定性显著降低(图 4),由此抑制了土壤有机酸的分泌,造成Citrate-P含量的增加。Enzyme-P代表了土壤中能被酸性磷酸酶和植酸酶矿化的有机磷[29],本研究中恩诺沙星添加前期Enzyme-P的含量变化并不显著,Low-ENR处理显著增加了土壤ACP的活性,而对ALP的活性则表现出抑制效果,二者的共同作用导致了Enzyme-P的含量变化并不显著。含phoD基因的细菌群落与土壤有机磷的矿化密切相关,其编码了ALP的分泌[30],低浓度恩诺沙星添加后土壤含phoD基因的细菌群落发生变化,其群落的复杂度显著降低,抑制了ALP的分泌,从而对土壤有机磷的矿化产生抑制作用。
-
抗生素的添加显著影响了微生物的丰度和组成。本研究中恩诺沙星添加后Rhodoplanes属和Pseudonocardia属的相对丰度显著降低(图 4)。之前的研究发现,在湿地生态系统中,氟喹诺酮类抗生素显著减少了变形菌门和厚壁菌门细菌的丰度,其中Dechloromonas和Delftia两个物种的相对丰度明显降低[31],与本研究结果相似。同时,微生物的群落网络特征也会受到抗生素的影响。本研究中恩诺沙星添加前期土壤含phoD基因的细菌群落平均度和模块性都显著下降(图 6)。文献[9]发现,水稻体系中阿维菌素添加后土壤中细菌和真菌的群落网络复杂性和稳定性均显著下降,与本研究结果相似。然而,也有研究表明,氟喹诺酮类抗生素增加了湿地生态系统中真菌和细菌的复杂性和网络互作,这可能与氟喹诺酮类药物的添加含量较低(50 μg/L)有关[32]。
微生物分泌的碱性磷酸酶是土壤中有机磷矿化的关键酶类。微生物群落特征的改变是影响相关酶分泌的重要原因,本研究中恩诺沙星的添加在前期显著抑制了土壤碱性磷酸酶的活性(图 3),这一结果在含phoD基因的细菌群落复杂度和稳定性的降低这一表现中得到了验证。另外,含phoD基因细菌的多样性和网络拓扑结构与土壤中SOC和TN密切相关[33],而本研究中土壤SOC和TN的综合变化使得恩诺沙星的添加对含phoD基因细菌的多样性影响并不显著(图 4)。土壤中Citrate-P模拟了可被有机酸活化和无机酸弱结合的无机磷,而pqqC基因是调控微生物溶解无机磷的关键基因,pqqC基因的变化可以通过Citrate-P的变化来验证[34]。恩诺沙星添加后,土壤含pqqC基因的细菌群落稳定性和多样性增加但复杂度降低,综合导致了土壤中Citrate-P含量的显著增加(图 2)。
抗生素的滥用和残留会对作物的生长产生抑制效果。本研究中,恩诺沙星添加后,土壤的磷酸酶活性增加,Enzyme-P含量显著增加,土壤的磷利用率增加,但是辣椒植株的吸磷量在恩诺沙星添加后显著降低,在文献[35]的研究中发现,土壤添加土霉素后,植株根鞘中与磷固定密切相关的羧酸盐酒石酸盐的含量降低,从而导致了植株磷吸收的减少。抗生素对植物的毒性作用是影响作物生长的又一个重要因素[36-37],有研究指出,高含量的抗生素会通过引起氧化应激来降低小麦的生长[38],同样在研究中发现磺胺嘧啶、四环素、恩诺沙星3种抗生素中,恩诺沙星对白刺生长的毒性作用最强[39]。说明恩诺沙星的添加虽然增加了土壤可利用磷的含量,但是抗生素本身对作物生长的毒性作用不可忽略,同时也会影响作物本身磷素的固定、转化等作用,从而抑制植株磷的吸收,并抑制作物的生长发育。因此,我们认为恩诺沙星在对土壤中磷的形态转化和有效性产生影响的同时,相应的毒性也会通过影响植物的代谢过程抑制作物的生长,两者之间存在权衡的作用,对作物的生长产生了负效应。
-
有机肥体系恩诺沙星的添加影响了磷循环微生物群落的结构和网络特征,在前期主要降低了含phoD基因的细菌的网络复杂度,抑制了土壤碱性磷酸酶的活性,从而影响土壤有机磷的矿化。同时,恩诺沙星的添加降低了土壤含pqqC基因的细菌群落的cohesion指数,其稳定性受到显著抑制,而群落的复杂度增加,影响了土壤无机磷溶解。本研究聚焦于磷循环功能基因phoD和pqqC,探究恩诺沙星的添加对土壤磷循环功能及辣椒生长的影响,为后续提高土壤磷素利用率,增加作物产量的研究提供了科学依据。






 DownLoad:
DownLoad: