-
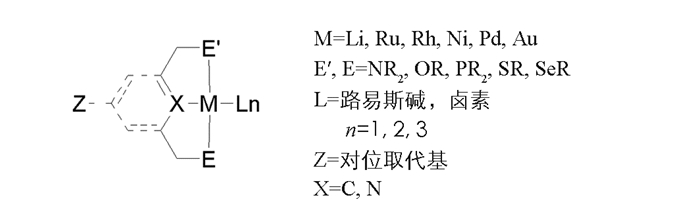
在日新月异的金属有机化学领域中,越来越多的钯化合物被合成出来并应用于各种反应中,其中pincer型环钯化合物由于具有高度的催化活性和刚性结构,吸引了众多化学家的兴趣,由此也推动了其在有机合成和催化领域中的飞速的发展[1]. pincer型配合物的结构示意图见图 1:M代表中心金属原子;E′,E代表不同的配位供电子基团;L代表配位的路易斯碱或是卤素原子;n代表配位数;Z代表对位取代基可以是供电子或是吸电子基团;X表示芳基或脂肪基上配位的原子一般为碳原子或是氮原子.
自从20世纪70年代Shaw等[2]和Van Koten等[3]报道Pincer型配合物以来,越来越多的Pincer型催化剂如(PCP型,NCN型,SCS型)被化学家们合成报道出来,应用于多种类型交叉偶联反应[4-5]构建C—B,C—O,C—N[6]等碳杂键,以及脱氢[7]、氢转移[8]和不对称催化[9]等反应中.
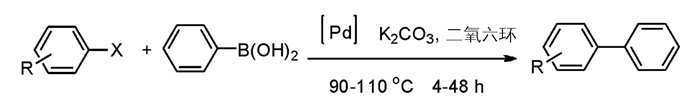
钯催化Suzuki偶联反应是金属有机反应中比较常见的反应,同时也是构建Aryl-Aryl中最常见的方法,Suzuki偶联反应在学术科研和工业生产中都有着广泛的应用[10].但钯催化剂具有对空气和湿度很敏感,不易操作和保存等缺点,而pincer钯催化剂能克服钯催化剂的以上缺点[11-14],由此pincer钯催化剂常被用于Suzuki偶联反应.但是目前对于pincer型钯催化的反应机理说法众多,有团队认为是配体脱离后进行反应[11],也有认为是先转金属化后再氧化加成[15],但对于本研究所采用的NCN pincer钯催化的反应机理鲜有报道[16].
通过NCN pincer钯配合物的初步催化活性筛选结果表明它能够有效催化Suzuki偶联反应(图 2),且已经对对反应条件进行了进一步优化,考察催化剂全面的性能和适用范围,并且通过单晶衍射确定了NCN-pincer配合物的化学空间结构[17-18].
HTML
-
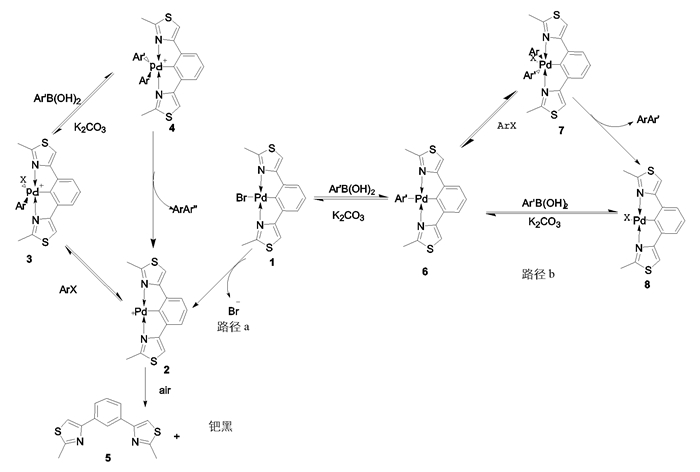
本研究采用密度泛函理论(density functional theory, DFT)的B3LYP泛函[19-20]对NCN-pincer钯催化Suzuki偶联反应机理进行了理论研究.使用高斯09软件包,采用密度泛函理论(DFT)的B3LYP泛函完成了所有计算.其中C,H,O,N,S采用6-31g*[21]基组进行计算,在化学实验中我们已经通过单晶衍射得到pincer钯配合物的空间排列结构,确定了计算中优化的结构模型.计算中对于反应中心元素Pd和卤素(Cl,Br,I)采用赝势基组LanL2DZ,并对Pd和卤素(Cl,Br,I)加了极化函数Cl(ζd= 0.514),Br(ζd= 0.389),I(ζd= 0.266),Pd(ζf= 1.472)[21],对所有计算的反应物、产物、中间体和过渡态都进行了振动频率分析,加入温度矫正函数T=383.15 k.为了使结果更为准确,对其过渡态进行IRC分析,证实过渡态是由反应物到生成物的能量最高点.提出了pincer-钯配合物应用于Suzuki交叉偶联反应以下两种可能的反应机理(图 3).
其中路径a:①物质1首先在溶剂高温条件作用下失去一个溴负离子配体,生成物质2(Pd正离子);②物质2再经过与卤带芳烃氧化加成,得到一个五配位的中间产物3;③物质3在碱性条件下与芳基硼酸转金属化生成物质4;④物质4还原消除释放出物质2和偶联产物,物质2催化下一循环过程.
路径b:①芳基硼酸在碱的作用下形成硼酸负离子,进而与物质1进行转金属化生成物质6;②物质6再与卤代芳烃进行氧化加成生成六配位的物质7;③物质7再还原消除得到偶联产物Ar-Ar’同时释放出pincer钯配合物8,而配合物8进入下一个催化循环.对决速步骤(氧化加成[15-16])的过渡态进行能量分析,进而讨论反应历程.
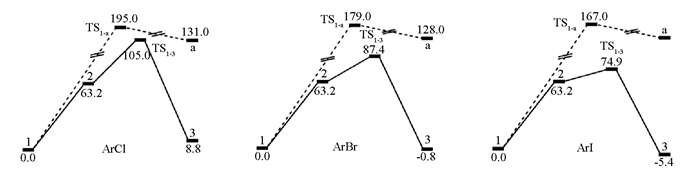
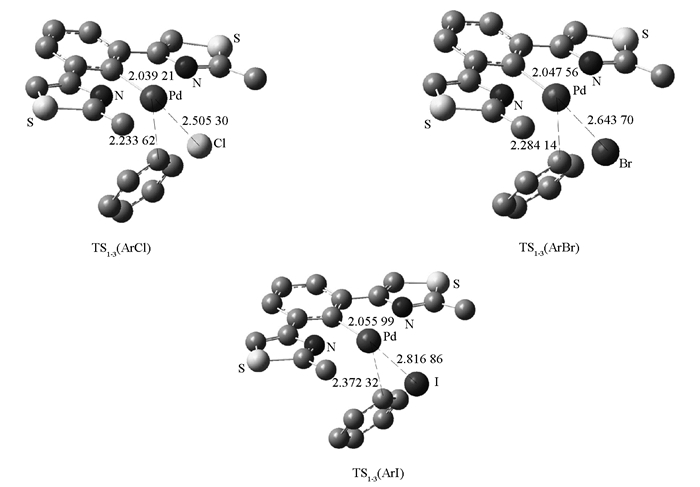
首先对Pincer钯配合物1直接与卤代芳烃氧化加成进行计算研究,得到物质1直接与卤代芳烃的氧化加成的能垒(图 4).
在计算中物质1直接对于碘苯、溴苯和氯苯进行氧化加成的能垒分别是167.0,179.0和195.0 KJ/mol.于是又对路径B进行了研究,计算发现物质6与碘苯的氧化加成的能垒都高达167.0 KJ/mol,那么显然物质6对于其他卤代芳烃的氧化加成的过渡态能垒会更高.于是又对路径A路径进行了研究,计算中发现当1失去一个配体溴再进行氧化加成时的能垒是比较低的,碘苯、溴苯分别是74.9,87.4 KJ/mol,对于氯苯则升高到了105.0 KJ/mol,认为能垒下反应是完全能够进行的.以上现象也解释了实验中物质1在催化溴代和碘代芳烃偶联时活性很高,而催化氯代芳烃的Suzuki交叉偶联反应产率普遍偏低[19].进一步对反应过渡态的空间化学结构进行研究发现,过渡态的化学结构中就键长从小到大顺序是:Pd—Cl键,Pd-Br键,Pd-I键,由于Pd—Cl键越短(图 5),氯代芳烃的氧化加成需要越高的能量,使得反应越难进行,产率越低,催化剂量需求越高.而对于碘代芳烃则反之.
在对不同的卤素取代基进行计算研究之后,又对芳基上的对位上取代基的电子效应进行了研究,计算发现甲氧基卤代芳烃的氧化加成的能垒要略高于对甲基取代的卤代芳烃,而甲氧基氯苯的氧化加成的能量很高,过渡态能垒高达108.0 KJ/mol,反之对甲基碘苯的氧化加成的过渡态能垒是75.3 KJ/mol,所以对甲基碘苯即使适当降低反应温度和催化剂用量都能有很高的收率,这些计算结果均与实验中发现的现象完全一致.即随着对位给电子的能力增强,偶联反应的能垒会逐渐升高,反应也就越难进行,对于ArX的能垒,则其从小到大的顺序是:ArI,ArBr,ArCl,也是与实验结果一致的[18].
-
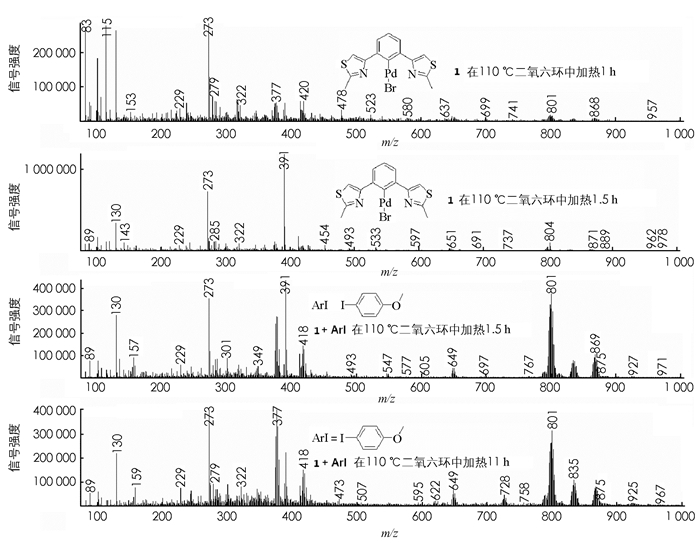
钯具有特殊的多种同位素峰,为了研究物质1在溶液中的变化以及添加碘代芳烃对催化剂结构的稳定性的影响,做了几个控制实验:①在100 mL圆底烧瓶中加入物质1 50 mg和50 mL二氧六环,110 ℃回流指定间隔时间内取样做MS分析;②在100 mL圆底烧瓶中加入物质1 50 mg和碘代芳烃100 mg,再加入50 mL二氧六环,110 ℃回流,相同间隔时间内取样做MS分析(图 6).
图 6表明:①添加了碘代芳烃的金属钯溶液中,钯配合物的寿命明显要比没有添加碘代芳烃的长,没有添加碘苯的钯溶液110 ℃回流1 h后,pincer钯配合物的MS峰就已经很少,搅拌反应1.5 h后几乎完全消失,表明pincer钯基本分解了;而加了碘代芳烃的钯配合物溶液,在同样的条件下加热到11 h后,钯配合物1的MS峰信号依然还很强,说明钯配合物1还是大量的以pincer钯配合物形式存在. ②pincer钯配合物1在催化过程中是伴随着碘代芳烃的消耗而慢慢分解.维持ArI /[PdII]适当高的比例有助于维持pincer钯配合物的寿命,增加底物与催化剂量的比值可以有效的提高催化剂的活性.在反应结束后发现1被分解成了5这种脱靶成氢的产物,没有监测到其他副产物的生成.
1.1. 计算部分
1.2. 实验部分
-
采用密度泛函理论的B3LYP泛函对NCN-pincer钯催化Suzuki偶联反应机理进行了理论研究,NCN-pincer钯对于ArX的氧化加成能垒从小到大依次是ArI,ArBr,ArCl. NCN-pincer钯先失去一个配体再参与了催化循环,与质谱实验结果一致的.证明了NCN-pincer钯催化Suzuki偶联反应经过了:①NCN-pincer钯失去溴;②氧化加成;③转金属化;④还原消除释放催化剂的过程.本研究采用密度泛函理论的B3LYP泛函和质谱实验对pincer钯催化Suzuki偶联反应机理进行揭示,通过双重验证保证偶联反应机理的准确性.同时机理研究也为实验化学家提供了设计更好催化剂的理论依据.






 DownLoad:
DownLoad: