-
干旱、水涝、极端温度和盐碱等非生物逆境胁迫严重影响着植物的生长、发育、产量和品质,是造成农作物和林果经济损失的主要环境因子.为了积极应对外界不良环境胁迫,植物在长期适应环境过程中进化出一系列防御机制,在接收逆境胁迫信号后,迅速启动逆境胁迫应答基因转录表达,调节植物体内物质代谢和信号转导,使植物产生内源抗性物质和形态变化,从而免受或减少不良环境造成的伤害[1].克隆、鉴定植物体内的逆境应答基因,不仅为鉴定抗逆种质资源提供理论基础,还可以为植物抗逆遗传改良工程提供优良基因,具有重要的理论意义和潜在的应用价值[2].
多胺(PAs)是植物体内广泛存在的一类低分子量脂肪族含氮碱,参与植物种子形成、器官发育、果实成熟和木质部形成等多种生理生化过程,还参与植物的多种生物和非生物胁迫应答反应[3-5].多胺是植物体内重要的逆境应答效应分子,其信号途径与植物体内多条代谢途径直接或间接相互作用,同时作为信号分子与植物体内复杂的激素信号网络相互交织,参与植物对逆境胁迫的应答反应[6].植物体内具有重要生理功能的多胺主要有腐胺(Put)、精胺(Spm)和亚精胺(Spd),腐胺合成主要涉及精氨酸脱羧酶(ADC)催化的间接途径和鸟氨酸脱羧酶(ODC)介导的直接途径,其中ADC途径能更有效地参与植物对逆境胁迫的应答反应过程[7].在模式植物拟南芥中,ADC基因有两个转录本AtADC1和AtADC2,其氨基酸存在80%的同源性,但它们的转录表达模式存在差异性,AtADC1基因在所有组织器官中低水平表达,而AtADC2基因主要在叶子、主根和果实中表达,同时受到不同非生物胁迫诱导表达[8-9].目前,ADC基因已从苹果、柑橘、葡萄、杧果和草莓等多种果树中克隆,并且该基因转录表达模式受到多种逆境胁迫不同程度的调控.干旱、低温等逆境胁迫诱导枳PtADC基因表达,超量表达PtADC基因的转基因材料显著提高了植株的抗旱性[10]; 盐胁迫能明显诱导苹果MdADC基因的表达,提高苹果愈伤组织多胺的含量,ADC途径积极参与了盐胁迫应答反应[7].通过转录组和代谢组学分析发现,StADC1介导的腐胺合成途径在马铃薯冷驯化耐寒性中起重要作用[11].杧果MiADC基因与枳精氨酸脱羧酶基因有较高的相似性,低温处理可诱导MiADC基因的转录表达[12].
桃树具有童期短、基因组小等特点,一直被作为研究果树重要农艺性状基因克隆和功能鉴定的模式材料[13].杂交育种、芽变和诱变育种等技术是桃树品种改良的主要途径,转基因技术在植物新品种培育和植株性状遗传改良中的优势,为桃树新品种选育和桃产业健康、快速发展提供了契机,发掘植物体内优良基因资源是顺利开展植物基因工程的必备条件[14-15].大久保桃作亲本培育的新品种,具有适应性广、抗性强、品质好等特点,是我国应用最广泛、培育品种数量最多、质量最高的优良亲本材料[16].随着桃树基因组学研究的进一步深入,应答逆境胁迫的优良基因陆续从桃树中获得克隆和鉴定[17].本研究以大久保桃为材料,在克隆桃树PpADC基因的基础上,对基因序列进行比对分析,通过低温、脱水、高盐和乙烯利等逆境胁迫处理,分析不同逆境胁迫下PpADC基因的表达模式,以期为深入阐述PpADC基因在桃树应答逆境胁迫过程中的功能提供理论基础.
HTML
-
植物材料采自华中农业大学桃园,桃树品种为大久保(Prunus persica L. Batsch cv Okubao).在8年生桃树相似部位剪取长度和形态一致的1年生嫩枝,健康无病虫害.剪取的嫩枝放入装有去离子水的三角瓶中,置于25 ℃,光周期16 h:8 h的光照培养箱培养1 d,然后进行脱水、盐胁迫、低温和乙烯利处理[18].脱水处理:将嫩枝置于干燥的三角瓶中,分别于0,0.5,1,3,6,12 h取样; 盐胁迫处理:将嫩枝置于200 mmol/L氯化钠(NaCl)溶液中,分别于0,1,5,12,24,48 h取样; 低温处理:将嫩枝插入含有去离子水的三角瓶中,置于4 ℃的培养箱中,分别于0,1,5,12,24,48 h取样; 乙烯利处理:将嫩枝插入含有去离子水的三角瓶中,喷施150 μL/L的乙烯利溶液,分别与0,0.5,1,3,6,12 h取样.
cDNA第一链合成试剂盒购自Invitrogen,Taq酶、荧光定量试剂盒等购自宝生物工程(大连)有限公司,胶回收试剂盒购自上海杰瑞生物公司,PCR引物由北京六合华大基因科技股份有限公司武汉分公司合成.
-
桃树嫩叶基因组DNA、总RNA提取参照改良的CTAB法[19-20],第一链cDNA合成参照试剂盒说明书进行.引物(PpADC-F,PpADC-R)用于PpADC基因克隆(表 1). PCR反应体系为20 μL: cDNA(总DNA)1 μL,引物(10 μM)各0.5 μL,dNTP(2.5 mM) 2.0 μL,Taq酶(5U/μL)0.2 μL,10×buffer 2.0 μL,ddH2O 13.8 μL. PCR反应条件: 94 ℃ 5 min,94 ℃ 30 s,56 ℃ 35 s,72 ℃ 2 min,35个循环,72 ℃ 7 min. 1%的琼脂糖凝胶电泳检测PCR产物,对目的基因片段进行切胶回收、纯化,连接pMD18-T载体,转化大肠杆菌DH5a感受态细胞,挑取阳性单克隆送北京六合华大基因科技股份有限公司武汉分公司测序.
-
利用NCBI的ORF Finder(https://www.ncbi.nlm.nih.gov/orffinder/)分析目标基因编码区; 用在线软件ProtParam(http://web.expasy.org/protparam/)对目标基因编码蛋白质量、等电点等进行预测分析; 用GOV Ⅳ(http://npsa-prabi.ibcp.fr)分析目标蛋白的二级结构; 用在线生物信息学软件SMART(http://smart.embl-heidelberg.de/)预测目标蛋白的功能结构域; 用在线软件DISPHOS(http://www.dabi.temple.edu/disphos/)预测目标蛋白的磷酸化位点; 不同物种同源蛋白序列的数据库检索利用NCBI的Blast搜索程序,多序列同源性对比分析由GENEDOC软件和NCBI提供的BLAST(https://blast.ncbi.nlm.nih.gov/Blast.cgi)在线软件程序完成,利用MEGA6.0软件构建ADC基因系统进化树.
-
应用qRT-PCR检测基因PpADC在低温、脱水、高盐和乙烯利等逆境胁迫处理过程中的转录表达量.样品RNA的提取及其cDNA的合成参照试剂盒说明书,在PpADC基因5′端非保守区设计基因特异引物(PpADC-QF,PpADC-QR),以桃树核糖体18 S基因为内参基因,其引物设计和序列参考RASORI等[21](表 1).扩增反应在LightCycler 480实时定量PCR仪(Roche Diagnostics)上完成,3次生物学重复,采用2-ΔΔCt方法计算不同处理材料中PpADC基因的相对表达量.
1.1. 材料及其处理
1.2. 基因克隆
1.3. 序列分析、编码蛋白预测及系统进化树构建
1.4. 基因表达模式分析
-
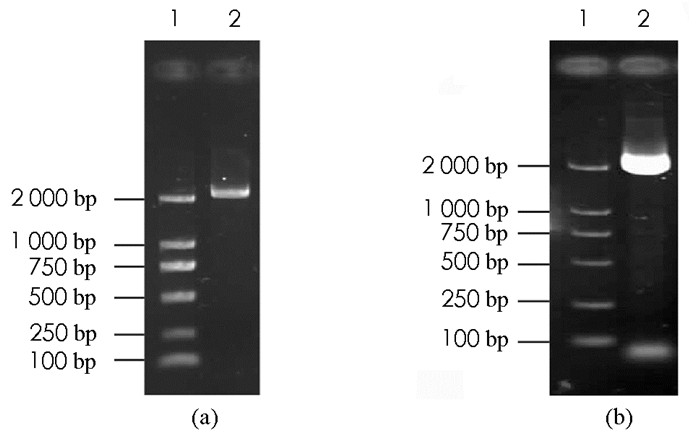
分别以桃树嫩叶总RNA反转录的cDNA和桃树基因组DNA为模板,以表 1中的基因扩增引物进行PCR,成功获得2个目的特异条带(图 1a、图 1b),由2次PCR产物的凝胶电泳图对比可知,2次PCR产物大小基本一致,片段长度大约为2.2 kb,与预期片段大小相近(图 1).测序结果显示,目的基因cDNA包含一条开放阅读框全长2 178 bp、编码725个氨基酸的核苷酸序列,用NCBI/BLAST在线程序进行同源性搜索,结果与扁桃ADC基因(VVA36875.1)同源性达到99.45%,将其命名为PpADC基因.将桃树嫩叶总RNA反转录的cDNA和桃树基因组DNA为模板的PCR产物的测序结果进行生物信息学分析,结果显示PpADC基因编码区不含内含子结构.
-
利用ProtParam在线软件对PpADC基因编码蛋白质序列的理化性质进行了预测.结果显示,PpADC基因编码的蛋白质分子量为77.7 kDa,理论等电点为5.19,蛋白的不稳定参数为43.11,脂溶指数为89.46,推测PpADC基因编码的蛋白质可能为亲脂性蛋白.
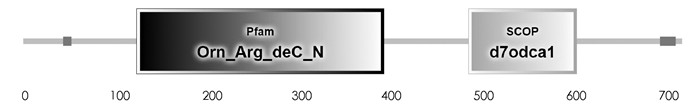
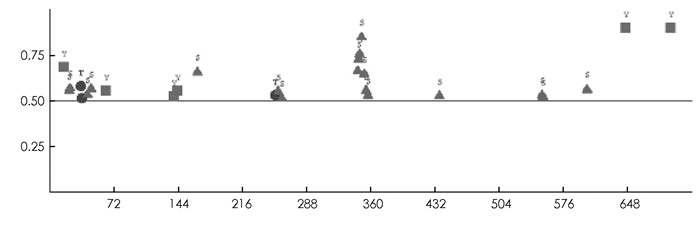
SMART预测结果显示,PpADC基因编码蛋白质N-端(44-53)和C-端(701-718)各有一个低复杂性结构域,中间包含一个具有催化活性的Ⅳ类脱羧酶结构域Orn_Arg_deC_N(125-397),一个SCOP结构域d7odca1(490-609)(图 2). GOR Ⅳ预测发现,PpADC编码的蛋白含有α-螺旋(Alpha helix)、延伸链(Extended strand)和无规则卷曲(Random coil)等结构,其中α-螺旋所占比例为37.66%,延伸链和无规则卷曲占比分别为15.59%和46.76%(图 3). DISPHOS预测结果表明,PpADC编码蛋白的丝氨酸、苏氨酸和络氨酸发生了磷酸化,丝氨酸磷酸化位点约占丝氨酸总数的26.09%,苏氨酸磷酸化位点约占苏氨酸总数的12.5%,络氨酸磷酸化位点约占络氨酸总数的25%(图 4).
利用NCBI数据库,将PpADC基因编码的氨基酸序列与其他物种ADC基因编码氨基酸进行比对,发现它们具有较高的相似性,其中与白梨PbADC基因编码氨基酸相似值为90.14%,与苹果MdADC基因编码氨基酸的相似值为89.84%,与湖北海棠MhADC基因编码氨基酸相似值为89.56%,与葡萄VvADC基因编码氨基酸的相似值为78.55%,与杧果MiADC基因编码氨基酸的相似值为74.49%,与枳PtADC基因编码氨基酸的相似值为73.58%,与拟南芥AtADC1和AtADC2基因编码氨基酸的相似值分别为74.28%和72.12%(图 5).
-
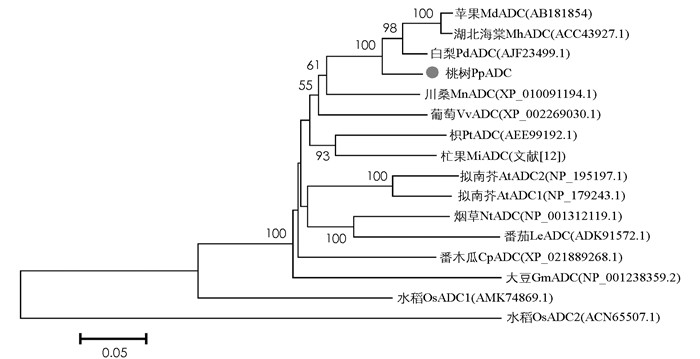
为了确定PpADC的系统进化位置,通过NCBI数据库选取了15个与PpADC蛋白同源性较高的其他物种的ADC氨基酸序列,利用MEGA6.0软件通过Neighbor joining(NJ)法,将PpADC与拟南芥、苹果、柑橘、白梨、葡萄、番茄、烟草等物种的ADC蛋白进行系统进化树分析.结果显示,PpADC基因与蔷薇科果树植物ADC基因亲缘关系较近,尤其是与白梨PbADC基因亲缘关系最近,与苹果MdADC基因、湖北海棠MhADC亲缘关系次之,而与模式植物拟南芥AtADC1,AtADC2和番茄LeADC(HM629957)亲缘关系相对较远,与水稻OsADC2基因亲缘关系最远(图 6),这表明ADC基因在蔷薇科植物中具有较高的保守性.
-
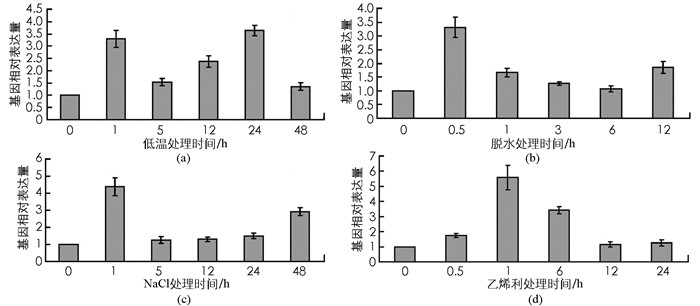
实时荧光定量PCR结果显示,PpADC基因转录表达受到低温、脱水、盐胁迫和乙烯利处理不同程度的诱导表达.低温处理明显诱导PpADC基因转录表达,分别在处理后1 h和24 h达到2个峰值(图 7a); 脱水处理后0.5 h,PpADC mRNA含量迅速积累并达到峰值,随后基因表达量降低并处于稳定水平(图 7b); 盐胁迫(200 mmol/L NaCl溶液)处理后1 h时,PpADC基因表达水平达到一个明显的峰值,相对于处理0 h提高了3倍左右,随后基因mRNA含量下降并维持低水平表达(图 7c); 乙烯利处理诱导PpADC基因表达量逐步积累,在处理后1 h达到峰值,随后基因表达水平呈现下降趋势(图 7d).
2.1. PpADC基因克隆及序列分析
2.2. PpADC基因编码氨基酸序列比对分析
2.3. PpADC蛋白的系统进化分析
2.4. 基因PpADC在逆境胁迫过程中的转录表达分析
-
多胺是植物体内一类低分子量脂肪族胺,广泛参与植物的生长发育和逆境胁迫应答反应[22].腐胺能积极应答多种逆境胁迫,提高植物应答低温、干旱和盐等逆境胁迫的能力,精氨酸脱羧酶(ADC)是腐胺合成的关键限速酶[23].桃树作为果树重要农艺性状研究的模式材料,是研究果树抗逆性的良好树种[24]. ADC基因编码的蛋白质是植物体内腐胺间接合成途径的关键限速酶,其最早从燕麦植物中获得克隆[25],本研究利用同源序列法克隆得到桃树精氨酸脱羧酶基因PpADC,该基因的开放阅读框全长为2 178 bp,编码725个氨基酸序列.桃树与柑橘、葡萄、苹果、草莓和杧果等其他果树具有相似的ADC基因序列,PtADC基因cDNA包含2 256 bp ORF,编码751个氨基酸序列[26],森林草莓FvADC基因ORF为2 154 bp,编码717个氨基酸序列[27],杧果MiADC基因cDNA含有2 178 bp的ORF,编码725个氨基酸序列[12]. ADC基因拷贝数在不同物种中存在差异性,ADC基因在桃树中只有1个拷贝PpADC,在拟南芥、番茄中却存在2个拷贝,但ADC基因结构在植物中具有高度一致性,PpADC基因结构中不含有内含子结构,与番茄SlADC1,SlADC2基因和杧果MiADC基因的结构类似[12, 28].
迄今,已有多种植物的ADC基因被成功分离、克隆,ADC转录水平的提高能有效增强植物对不良环境的抗逆性,但ADC基因在多种逆境胁迫条件下的转录表达模式较为复杂[29].本研究中,低温、脱水、盐胁迫和乙烯利处理均能诱导PpADC基因转录表达,但PpADC基因在应答不同处理过程中的转录表达模式有所差异,脱水和盐胁迫处理后PpADC基因表达量快速积累,然后维持在较低水平表达,低温处理诱导PpADC基因表达维持在高水平状态,乙烯利处理导致PpADC基因表达量前期缓慢积累,后期缓慢下降.与桃树PpADC基因相似,ADC基因在应答逆境胁迫过程中的转录调控模式在其他植物中也得到证实.杧果MiADC基因转录表达受到低温处理影响,是低温胁迫应答反应的候选基因[12]; 枳PtADC基因转录水平受到低温和脱水处理上调表达[26].苹果低温、盐和脱水处理能诱导MdADC基因转录表达,表明该基因参与了苹果的逆境胁迫应答反应[30].水稻OsADC1基因转录表达受到低温处理诱导,但盐和干旱处理2 d不影响OsADC1基因转录表达[31].植物ADC基因转录表达受到多种逆境胁迫调控,有力地支撑了多胺广泛参与植物逆境胁迫应答反应,但不同植物ADC基因在应答逆境胁迫过程中的不同调控模式,表明该基因应答逆境胁迫的机制存在物种差异性. ADC基因在植物中拷贝数不同,同一物种的不同ADC基因在逆境胁迫过程中的表达模式存在差异性.番茄植物中,高温和H2O2处理能诱导基因LeADC1和LeADC2转录表达,伤害和低温处理仅能诱导基因LeADC2转录表达[28].低温处理能显著诱导芥菜MADC1,MADC2和MADC3基因的转录表达,盐胁迫处理诱导MADC3基因转录水平增加,却不影响MADC1,MADC2基因表达,外源多胺处理刺激MADC2和MADC3基因mRNA的积累,但不影响MADC1基因表达[32].拟南芥AtADC1基因受到细菌感染的特异性诱导,但AtADC2基因对植株体内ADC总活性具有重要贡献,AtADC1和AtADC2在一定程度上的功能冗余对植物基础抗性有明显贡献[33].基于PpADC基因在低温、脱水、高盐和乙烯利处理过程中的转录表达模式,笔者推测桃树PpADC基因通过感受外界不良环境的诱导表达,PpADC基因mRNA的积累导致桃树体内ADC蛋白含量增加,从而提高ADC对腐胺间接合成的催化作用,增加植物体内多胺含量,减少逆境胁迫对植物造成的伤害.然而PpADC基因转录表达在不同逆境胁迫条件下的差异性调控,以及同一物种的不同ADC基因拷贝在相同逆境胁迫条件的转录调控差异性,充分表明了ADC基因应答逆境胁迫的复杂性,以及逆境胁迫对ADC基因转录调控机制的多样性.
本研究克隆到桃树PpADC基因,该基因无内含子结构,与蔷薇科植物ADC基因具有较高的同源性,低温、脱水、盐和乙烯利等逆境处理不同程度地诱导PpADC基因转录表达,表明PpADC基因在桃树应答非生物逆境胁迫过程中发挥重要作用.







 DownLoad:
DownLoad: