-
固态法酿造的中国白酒是中国独有的蒸馏酒,拥有2000多年的酿造和饮用历史[1-2]. 白酒是中国传统的发酵食品,不仅蕴含了厚重的中国历史和文化,还富含有众多的风味成分[3-4]. 由于酒精摄入人体后主要通过肝脏进行代谢,肝损伤在饮酒带来的健康问题中占有重要比例,尤其是酒精性脂肪肝. 有研究认为在适度饮用固态法酿造的白酒时,其中含有的多种风味物质可以在一定程度上降低酒精对人体的伤害,因此其对人体健康的影响与单纯的酒精不同[5-6]. 目前关于白酒对健康影响的研究文献多着眼于分析白酒风味物质中的活性成分,通过活性成分本身的功能推测其在白酒中可能起到的对健康的影响[7-8]. 本研究使用基于小鼠肝实质细胞(AML12)的肝脂质聚集模型[9],对比研究几种浓香型川酒与单纯酒精对肝脂质代谢的影响,检测分析这几种川酒的主要风味物质,并对主要挥发性成分的差异用统计学方法进行分析,研判其是否具有显著性,结果将为白酒中的风味物质对肝脏脂质水平的影响提供数据和研究模型.
HTML
-
四川某品牌浓香型白酒A(酒精浓度为52%),四川某品牌浓香型白酒B(酒精浓度为52%),四川某品牌浓香型白酒C(酒精浓度为52%).
所用试剂及药品购于美国Sigma Aldrich公司,实验用水皆为去离子水.
气相色谱质谱联用仪(GC-MS联用仪6890N-5975B inert XL EI/CI MSD),美国Agilent公司; 正倒置一体显微镜(Revolve G176),美国Echo公司.
-
准确量取900 μL酒样,并加入100 μL 2-辛醇内标溶液,将顶空进样瓶密封,直接自动进样.
-
Agilent J&W DB-WAX气相色谱柱(122-7062),柱长60 m,内径250 μm,膜厚度0.25 μm. 升温程序: 起始温度38 ℃,以1 ℃/min升温至60 ℃,保持2 min; 以3 ℃/min升温至70 ℃,保持2 min; 以4 ℃/min升温至105 ℃,保持2 min; 以4 ℃/min升温至180 ℃,保持1 min; 以6 ℃/min升温至230 ℃,保持2 min. 汽化室温度250 ℃; 载气He,柱前压15.92 psi,载气流量1 mL/min,进样量1 μL; 不分流进样.
-
质谱的电离方式为电子轰击离子源(EI). 离子源温度230 ℃,四极杆温度150 ℃,电子能量70 eV,发射电流34.6 μA,倍增器电压1 470 V,进样口温度230 ℃,扫描质量范围20~550 amu,溶剂延迟3 min.
-
用血球计数板法测定小鼠正常肝细胞(AML12)浓度,取35 mm培养皿,每个培养皿里接种1× 105个肝细胞,放置培养箱中(37 ℃,5% CO2)培养24 h. 然后将肝脏细胞样品分别进行如下处理: ①脂质处理组,只用棕榈酸和油酸处理细胞; ②四川浓香型白酒处理组: 分为3个不同白酒处理组,在脂质处理的基础上分别加入3种不同品牌的浓香型白酒A,B和C. 每个白酒处理组又分为两个不同剂量亚组,即在培养液中最终酒精浓度为1%和0.5%; ③酒精处理组: 该组在脂质处理的基础上添加纯酒精并分为两个剂量亚组,分别是在培养液中最终酒精浓度为1%和0.5%; ④无处理空白对照组: 该组不进行脂质、添白酒和纯酒精处理. 细胞进行各种处理后在培养箱中(37 ℃,5% CO2)培养24 h,然后采用尼罗红(染色细胞脂滴中的中性脂)和Hoechst33342(染色细胞核)对细胞内的脂滴和细胞核同时避光进行双染色20 min,用Revolve G176正倒置一体显微镜和ImagJ软件使用预先设定的方法测定肝脏细胞内被尼罗红染色脂滴的荧光强度和Hoechst33342染色的肝脏细胞核数,并计算每个肝脏细胞的平均脂滴荧光强度.
-
脂质处理组、各浓香型白酒处理(亚)组、酒精对照(亚)组、空白对照组均设置3个平行样品,实验进行3次重复,取3次重复实验平均值(x±s)作为实验结果. 白酒酒样的检测进行3次独立实验,取3次实验平均值(x±s)作为实验结果. 对比各处理组之间细胞脂质水平差异使用One way ANOVA,各处理组1%和0.5%亚组之间细胞脂质水平差异使用双尾Students’T test,白酒A和B之间风味物质差异使用双尾Students’T test进行统计学分析.
1.1. 材料、试剂与仪器
1.2. 方法
1.2.1. 顶空固相微萃取(headspace-SPME,HS-SPME)方法
1.2.2. 色谱(GC)条件
1.2.3. 质谱(MS)条件
1.2.4. 肝脏细胞平均脂滴荧光强度
1.3. 数据统计
-
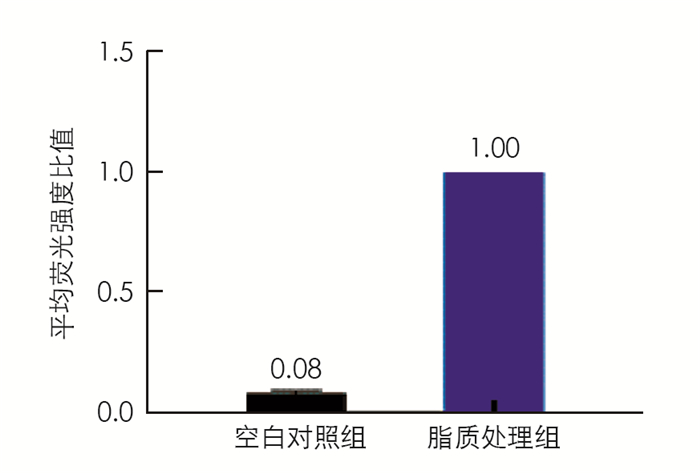
本研究基于油酸和棕榈酸处理肝细胞造成细胞脂质聚集模型[10]检测不同药物处理对肝细胞内脂质的聚集水平,实验结果显示脂质处理组的细胞中脂质水平是空白对照组细胞的12.5倍,表明细胞中形成了脂质聚集(图 1).
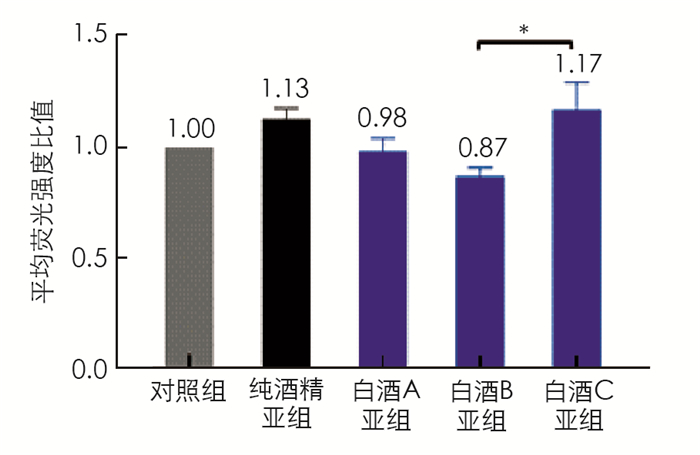
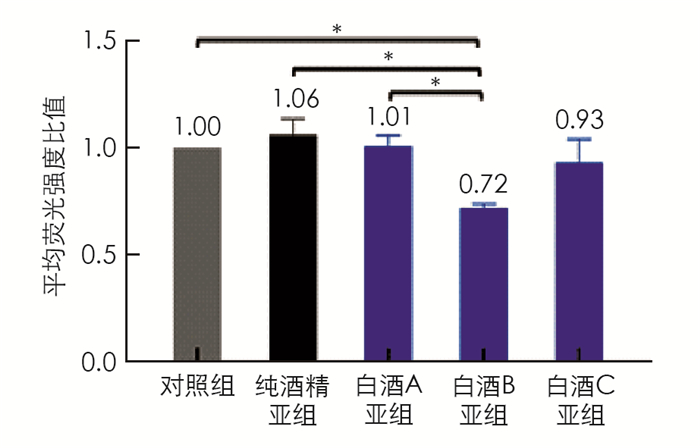
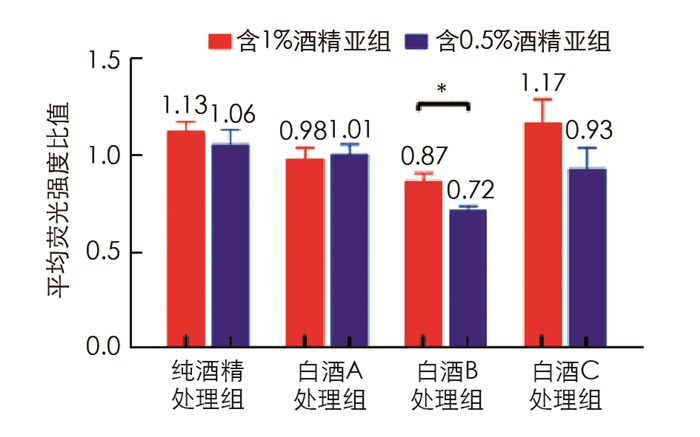
对脂质聚集的细胞分别用酒精或3种不同浓香型川酒进行处理,含1%酒精处理亚组中,白酒B亚组的脂质水平为0.87,显著低于白酒C亚组的脂质水平(1.17,p < 0.05); 脂质处理组(以下简称对照组)的脂质水平要低于纯酒精亚组,白酒A亚组和白酒B亚组细胞内脂质也低于纯酒精亚组,但差异无统计学意义(图 2). 含0.5%酒精的处理亚组中白酒B亚组的脂质水平(0.72)显著低于对照组(p < 0.05)、纯酒精亚组(p < 0.05)和白酒A亚组(p < 0.05),其他各组的脂质水平差异无统计学意义(图 3). 同种处理的亚组中,除白酒A外,含0.5%酒精的亚组的脂质水平均要低于含1%酒精的亚组(图 4),其中白酒B含0.5%酒精亚组的脂质水平显著低于含1%酒精亚组的脂质水平(p < 0.05).
-
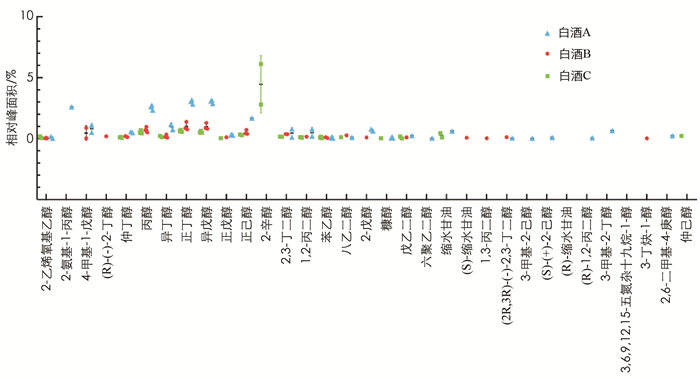
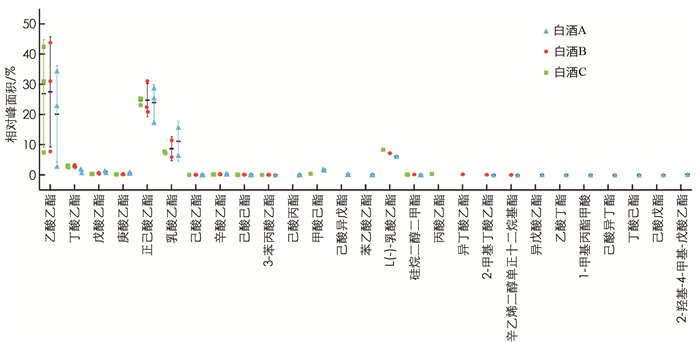
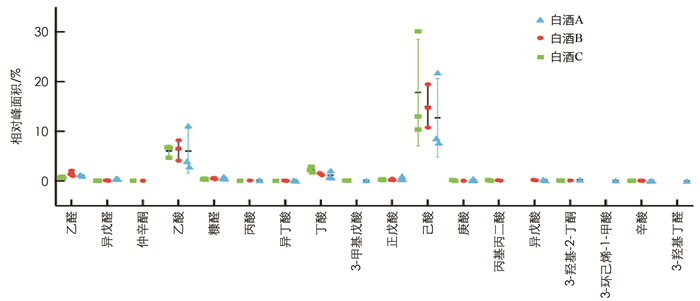
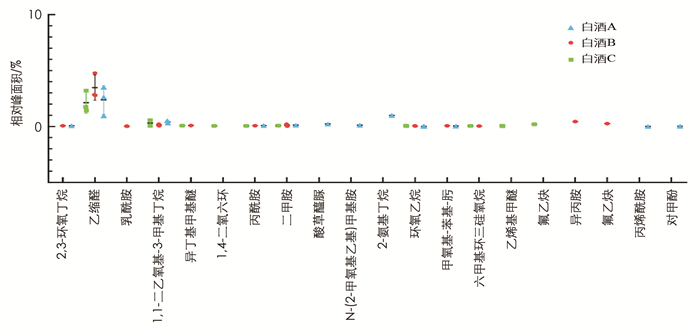
通过GC-MS分别从3种浓香型白酒中检测出的风味物质较丰富,有33种醇类(图 5)、27种酯类(图 6)、18种醛类、酮类和酸类(图 7)以及20种其他物质如乙缩醛等(图 8). 3种白酒虽然同是浓香型白酒,但在醇、酯、酸和醛等风味物质的种类和相对浓度上还是有一定的差异.
首先,含0.5%酒精的白酒B亚组中脂质水平显著低于白酒A亚组(p < 0.05),而与白酒C亚组差异无统计学意义,因此对白酒A和B中均含有的挥发性风味物质(相对浓度1%或以上)进行分析比较,结果显示其相对浓度有一定的差异(表 1). 如在醇类物质中,白酒A中检测到明显高于白酒B的丙醇(分别为2.57%和0.73%,p < 0.001)、正丁醇(分别为3.02%和1.00%,p < 0.001)和异戊醇(分别为3.04%和0.99%,p < 0.001). 两种白酒都含有较高的乙酸乙酯、正己酸乙酯和乳酸乙酯,但差异均无统计学意义. 其中正己酸乙酯在两种白酒中的相对浓度差异不大,而白酒B中的乙酸乙酯(27.52%)要高于白酒A(20.14%),白酒B中乳酸乙酯(8.65%)要低于白酒A(11.16%). 乙酸和己酸在两种白酒的挥发性风味物质中的比重也较高,但差异无统计学意义. 乙缩醛在白酒B中的相对浓度(3.47%)要高于白酒A(2.38%),但差异无统计学意义.
此外,3种白酒均含有其特有的,即其他两种白酒中未检测到的挥发性风味物质. 如白酒C含有4.46%的2-辛醇,而在白酒A和B中未检出; 白酒A含有在白酒B和C中未检出的己酸丙酯(0.07%); 异丁酸乙酯只在白酒B中有检出(0.18%).
还有个别风味物质如4-甲基-1-戊醇只在白酒A与B中有检出,甲酸己酯只在白酒A与C中有检出,而仲辛酮只在白酒B和C中有检出.
2.1. 肝细胞内脂质聚集水平差异
2.2. 挥发性风味物质成分
-
本研究发现两个不同剂量的纯酒精处理亚组和白酒A与C亚组的细胞脂质水平与对照组差异无统计学意义. 白酒B(含0.5%酒精)亚组的细胞脂质水平是各组中最低的,且显著小于对照组(p < 0.05)和0.5%纯酒精亚组(p < 0.05),这很有可能是由白酒B中非酒精组分造成的; 白酒B(含0.5%酒精)亚组的细胞脂质水平显著低于白酒A(含0.5%酒精)亚组(p < 0.05),进一步表明两组脂质水平的差异可能是由白酒B与白酒A中有差异的非酒精成分造成的. 对比白酒A与白酒B中主要挥发性风味物质发现,差异有统计学意义的风味物质相对浓度在白酒A中均高于白酒B. 而相对浓度超过5%的乙酸乙酯、正己酸乙酯、乳酸乙酯、乙酸和己酸(乳酸乙酯除外)在白酒B中相对浓度均高于白酒A,但差异无统计学意义. 这表明白酒B与白酒A对细胞脂质水平影响的差异可能不是由某一种相对浓度有差异的风味物质造成,而可能是由两种或以上的风味物质共同作用造成,其中也有可能包含有相对浓度较高的非挥发性物质.
纯酒精、白酒B和白酒C处理组中均呈现出随酒精相对浓度从1%减低到0.5%其脂质水平降低的趋势,尤其是白酒B,脂质水平降低有统计学意义(p < 0.05),这表明,酒精本身对细胞脂质水平存在影响.
本研究使用的模型是脂肪肝的细胞模型[10-12],脂质代谢是肝脏的重要功能之一[13]. 肝脏脂质代谢紊乱会引起脂肪肝,脂肪肝的病理起源除了长期酗酒外[14],还有一类是由于生活和饮食习惯引起的非酒精性脂肪肝[15-16],中国目前有近30%的人群是非酒精性脂肪肝患者[17],因此研究饮酒对非酒精性脂肪肝的影响及其机理也是十分必要的.
-
肝细胞的脂质代谢和其脂质水平与人体健康息息相关[18]. 本研究使用基于AML12肝细胞的脂质聚集模型初步探究了3种不同浓香型白酒对肝细胞内脂质水平的影响,结果显示白酒B对肝细胞脂质水平的降低可能是由两种或以上主要挥发性风味物质组合(可能也包括浓度较高的非挥发性物质)造成. 接下来的研究一方面进一步测试主要风味物质单独和组合对肝细胞脂质水平的影响,探究其影响的细胞代谢路径; 另一方面使用GC-MS联用检测并比较非挥发性风味物质.






 DownLoad:
DownLoad: