-
开放科学(资源服务)标识码(OSID):

-
目前,植物病害主要通过化学农药进行防控,该方法虽然耗时短、见效快,但存在易导致环境污染、使植物病原菌产生抗药性等问题[1-2]. 因此,开发对环境绿色安全且能持久有效地防治植物病害的生物防控方法具有重要意义. 其中,益生微生物制剂就是化学杀菌剂的良好替代品[3]. 自1921年Hartely首次利用拮抗真菌防治Pythium debaryanum引起的猝倒病以来,部分益生真菌、放线菌和细菌等微生物被广泛应用于生物防治[4-5]. 芽孢杆菌是土壤和植物相关微生物的优势种群,具有较好的抑制植物病原菌的能力,对环境无毒害作用,且其芽孢抗逆性强,被广泛用于植物病害生物防治[6]. 其中,贝莱斯芽孢杆菌(Bacillus velezensis)是2005年才被报道的一种新型生防菌种[7]. 2016年Dunlap等[8]通过比较基因组学和in silico DNA-DNA杂交手段,将甲基营养型芽孢杆菌(B. methylotrophicus)、解淀粉芽孢杆菌植物亚种(B. amyloliquefaciens subsp. plantarum)和稻生芽孢杆菌(B. oryzicola)归类为贝莱斯芽孢杆菌. 研究表明贝莱斯芽孢杆菌是极具应用潜力的生防细菌,Liu等[9]分离出的B. velezensis D4可以抑制苹果树腐烂病病原菌黑腐皮壳菌(Valsa mali)的生长,分离自番茄根际土壤的B. velezensis Y6菌株对植物病原菌尖孢镰刀菌(Fusarium oxysporum)孢子萌发表现出较强的抑制作用[10].
芽孢杆菌的抑菌机制主要包括拮抗作用、竞争作用和诱导植物抗性等[11]. 拮抗作用是指微生物产生拮抗物质直接抑制或杀死另一种微生物. 芽孢杆菌可产生脂肽类化合物、聚酮类化合物和抗菌蛋白等拮抗物质抑制病原菌生长[12]. 其中,脂肽类化合物由非核糖体肽合成酶(nonribosomal peptide synthetase,NRPS)途径合成,而聚酮类化合物则是由聚酮合酶(polyketide synthase,PKS)途径合成[12]. 脂肽类化合物主要包括3个家族:表面活性素(Surfactin)、丰原素(Fengycin)和伊枯草菌素(Iturin),这类化合物具有很强的抗菌活性[13-14],其主要作用于病原菌细胞膜或细胞壁[15-17]. 李生樟等[18]研究报道B. velezensis 504菌株基因组含有编码脂肽类和聚酮糖类抑菌化合物的基因簇,并能够特异性拮抗黄单胞菌;Jin等[19]报道了B. velezensis S3-1菌株可产生属于丰原素、伊枯草素和表面活性素家族的13种脂肽类抗生素,且能抑制植物病原菌Botryis cinerea生长.
本研究以植物炭疽病菌Colletotrichum sp.为靶标菌,对一株具有广谱抑菌活性的拮抗B. velezensis SWUJ1[20]进行全基因组测序,并对其抑菌物质进行分离纯化,初步探究其抑菌机理,为该菌进一步开发成生防制剂奠定前期研究基础.
全文HTML
-
供试植物炭疽病菌Colletotrichum sp.和拮抗菌株B. velezensis SWUJ1为本实验室收集保存[20].
PDA培养基(g/L):土豆200.0,葡萄糖20.0,琼脂15.0~20.0;PDB培养基(g/L):土豆200.0,葡萄糖20.0;LB培养基(g/L):胰蛋白胨10.0,酵母粉5.0,NaCl 10.0,琼脂15.0~20.0;改良PDB培养基和Landy培养基分别参考文献[20]和文献[21]配制.
-
植物炭疽病菌Colletotrichum sp.和拮抗细菌B. velezensis SWUJ1活化的具体方法为:取甘油保种的真菌菌饼和细菌菌液分别接种至新鲜的PDA和LB培养基上进行活化,真菌于25 ℃培养7 d,细菌于28 ℃培养1 d. 采用平板对峙法[22]检测抑菌活性:将B. velezensis SWUJ1新鲜培养物接种于PDB培养基,28 ℃摇床180 r/min振荡培养24 h,获得菌悬液;用直径为5 mm的打孔器在长满新鲜植物炭疽病菌Colletotrichum sp.菌丝的PDA平板边缘取菌饼,接种至新的PDA平板中央,同时在距离病原菌边缘30 mm的对称处划线接种新鲜拮抗细菌,以仅培养病原菌的PDA平板为对照,各设3个重复,25 ℃培养9 d后测量病原菌的菌落直径,计算抑菌率. 用灭菌牙签挑取培养9 d对照组病原菌正常生长菌丝,以及处理组处于抑菌带边缘的病原菌菌丝制片,在光学显微镜下观察病原菌菌丝形态. 菌落直径和抑菌率的计算公式为:
-
挑取新鲜B. velezensis SWUJ1单菌落接于LB液体培养基,37 ℃摇床培养16 h,8 000 r/min离心10 min收集菌体,参照Qiagen试剂盒的方法进行基因组DNA的提取. 样品纯度(A260/280处于1.8~2.0之间)、质量浓度(>100 ng/μL)检测合格后,送至武汉未来组生物科技有限公司进行全基因组测序.
-
利用Oxford Nanopore Technology测序仪GridION对DNA进行单分子测序,建库完后将一定浓度和体积的DNA文库加入到流通池中,并将其转移到GridION测序仪进行实时单分子测序,获得原始数据,并对质控后的数据进行基因组组装、矫正及优化.
-
编码基因用prodial进行预测,保留完整的编码序列(CDS). 提取基因组编码蛋白后,用Interprosca进行注释,提取GO数据库的注释信息;用blastp比对编码蛋白到KEGG数据库,保留比对覆盖度大于30%的最好结果作为注释结果;用rpsblast比对编码蛋白到COG数据库进行注释. 采用在线预测软件antiSMASH 5.0.0(https://antismash.secondarymetabolites.org/#!/start)对菌株SWUJ1次级代谢产物合成基因簇进行预测分析,寻找潜在的抑菌代谢产物合成基因簇.
-
将新鲜B. velezensis SWUJ1菌株接种于LB液体培养基,28 ℃、180 r/min振荡培养16 h,获得新鲜种子液. 将SWUJ1菌株种子液按1%的接种量接种至改良PDB培养基,于25 ℃及180 r/min条件下培养4 d,将菌悬液在8 000 r/min条件下离心20 min,弃菌体沉淀,取上清过0.22 μm滤膜,得到无菌发酵滤液;分别用40,60,80,100 ℃金属浴热处理发酵滤液30 min,冷却至室温,以未做热处理的发酵滤液为对照,检测各组滤液的抑菌活性;采用抑菌圈法[23]检测发酵液抑菌活性:用直径为5 mm的打孔器在长满新鲜植物炭疽病菌Colletotrichum sp.菌丝的PDA平板边缘处取菌饼,接种至新的PDA平板中央. 25 ℃培养2 d后,在试验平板上距病原菌边缘15 mm处,用直径为5 mm的无菌打孔器打孔,往孔中注入80 μL无菌发酵滤液. 每个处理设置3个重复,25 ℃培养2 d后,测量抑菌带大小.
-
基于拮抗菌株的全基因组分析和发酵滤液的热稳定性,推测该菌活性产物可能为脂肽类抗生素,故选用芽孢杆菌产脂肽常用的Landy培养基作为发酵培养基,利用发酵罐进行菌株体外扩大培养,具体发酵条件为:50 L发酵罐,装量31 L,接种量为10%,25 ℃,pH值为7.0,300 r/min搅拌培养3 d后,8 000 r/min离心20 min,取发酵上清液备用.
-
采用酸沉淀法[24]提取活性发酵液中的抑菌物质,具体方法为:用6 mol/L浓HCl将1.5.1制备的发酵上清液的pH值调至2.0,4 ℃静置沉淀12 h;4 ℃,8 000 r/min离心20 min收集沉淀,待沉淀自然干燥后加适量甲醇溶解;多次抽滤至滤液无活性,合并滤液,旋转蒸发浓缩粗提物.
-
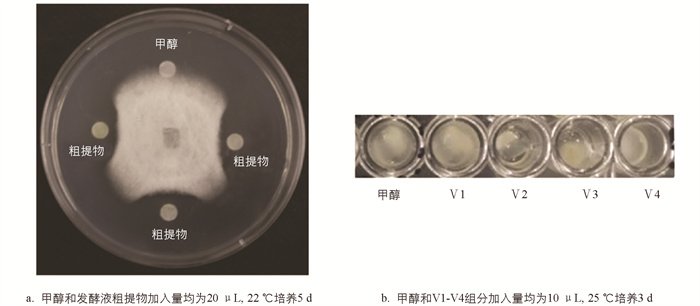
采用纸片抑菌圈法[23]检测发酵液粗提物对炭疽病菌的抑菌活性,具体方法为:将新鲜病原菌菌饼接种在PDA平板中央,并在距离菌饼中心20 mm处放置无菌滤纸片,在滤纸片上加入20 μL发酵液粗提物,以滴加等量甲醇为空白对照,22 ℃培养5 d,观察是否形成抑菌带,以此判断发酵液粗提物的抑菌活性.
-
对获得的发酵液粗提物进行LC-MS分析,具体方法参考文献[25]:LC-MS分析使用Agilent 1290 Infinity Ultra Performance Liquid Chromatography与Agilent 6545 UHD and Accurate-Mass Q-TOF质谱联用仪. 色谱柱型号为Waters XSelect© HSS T3柱(2.5 μm,100 mm×2.1 mm);流动相:A为水溶液(0.1%甲酸),B为乙腈(0.1%甲酸);流速设置为0.35 mL/min,柱温为40 ℃. 进样量:正离子模式1 μL,负离子模式2 μL. 优化的色谱梯度:0~2 min,5% B;2~10 min,5%~95% B;10~15 min,95% B. 运行时间设为5 min,用于平衡系统. 质谱使用正离子模式结合负离子模式,优化的参数如下:毛细管电压为3.5 kV;干燥气流为10 L/min;气体温度为325 ℃;喷雾器压强为1 363.9 kPa;碎裂电压为120 V;取样锥电压为45 V. 质谱的采集范围50~3 000 m/z,数据系统分析使用Agilent Masshunter Qualitative Analysis B. 08.00 software(Agilent Technologies,USA).
-
采用C18反相柱层析对发酵粗提物进行分离,以20%,40%,60%,80%的甲醇进行梯度洗脱,流速为20 mL/min,分别收集各梯度洗脱液,浓缩后用纸片抑菌圈法检测各分离组分对炭疽病菌的抑菌活性. 用直径为0.22 μm的滤膜对活性较好的组分进行过滤,利用汉邦高效液相色谱仪,以YMC-Pack ODS-A液相色谱柱(C18柱)进一步分离纯化抑菌活性组分. 以水为流动相A,乙腈为流动相B,按表 1方法进行分离纯化,浓缩后用纸片抑菌圈法检测各分离组分对炭疽病菌的抑菌活性. 利用Sephadex LH-20凝胶层析对活性较好的组分进行分离,洗脱剂为甲醇,每5 mL收集一个组分,各组分通过薄层色谱(TLC)点板合并,以炭疽病菌为指示菌,采用96孔板法测定各合并组分的抑菌活性,具体方法为:在96孔板的小孔内各自加入100 μL PDB培养基. 对照组中加入10 μL甲醇,处理组中加入10 μL分离组分,25 ℃培养3 d,观察炭疽病菌生长情况.
-
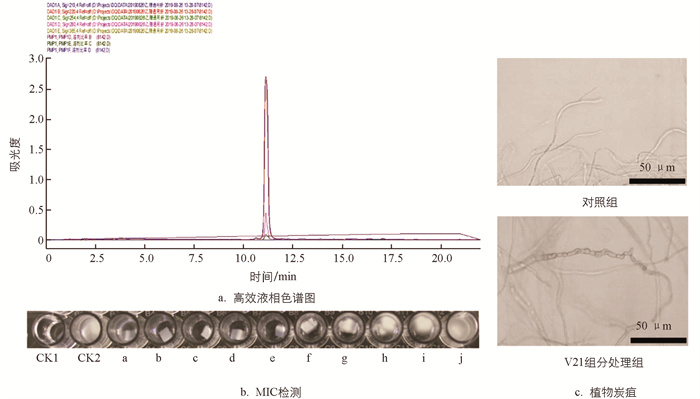
取5 mg分离获得的单峰物质溶于1 mL的甲醇溶液,配制成5 000 μg/mL的抑菌溶液. 在96孔板中分别加入抑菌溶液,待其风干后,加入100 μL PDB培养基,每孔样品质量浓度依次为500,450,400,350,300,250,200,150,100,50 μg/mL. 每孔加入大小均一的Colletotrichum sp.菌块,以甲醇及PDA块为对照,25 ℃培养3 d,观察真菌的生长情况,确定真菌完全被抑制时的最低抑菌浓度(MIC),实验平行重复3次,并在光学显微镜下观察活性化合物对病原菌菌丝形态的影响.
1.1. 供试材料与培养基
1.2. 菌株活化及抑菌效果检测
1.3. 拮抗菌株B. velezensis SWUJ1全基因组测序
1.3.1. 拮抗菌株基因组DNA的提取
1.3.2. 基因组DNA测序与组装
1.3.3. 基因预测与功能注释
1.4. 拮抗菌株B. velezensis SWUJ1发酵滤液热稳定性检测
1.5. 拮抗菌株B. velezensis SWUJ1发酵液粗提物的制备
1.5.1. 菌株活性发酵上清液的制备
1.5.2. 活性物质的粗提
1.5.3. 抑菌活性的检测
1.6. 拮抗菌株B. velezensis SWUJ1发酵液脂肽类抑菌物质的分离鉴定
1.7. 拮抗菌株B. velezensis SWUJ1发酵液小分子抑菌物质的分离纯化
1.8. 活性物质最低抑制质量浓度的测定及其对病原菌菌丝形态的影响
-
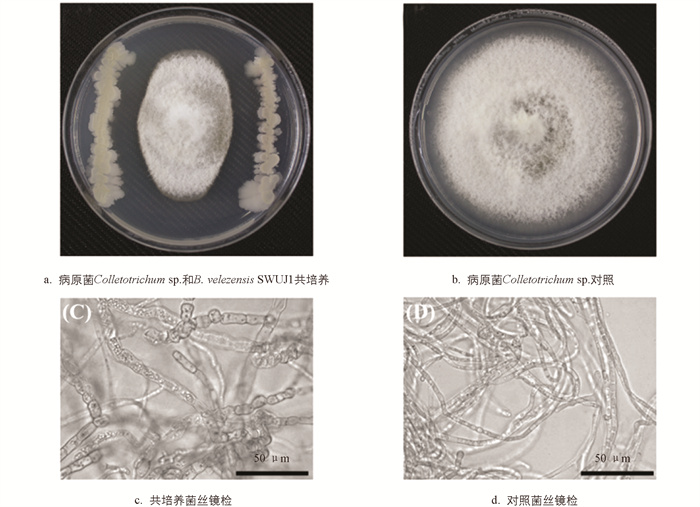
平板对峙实验结果显示,B. velezensis SWUJ1对供试炭疽病菌生长具有较好的抑制作用(图 1a),其抑菌率可达58.90%,而对照组病原菌菌丝长满整个平板(图 1b). 对不同组真菌菌丝进行显微观察,结果表明B. velezensis SWUJ1处理组的炭疽病菌部分菌丝扭曲、畸形、肿大,且内容物聚集(图 1c),而对照组真菌菌丝无色透明、细长、光滑匀整(图 1d),说明B. velezensis SWUJ1菌株对病原菌菌丝有明显的破坏作用.
-
B. velezensis SWUJ1菌株全基因组测序结果表明,该菌染色体全长为3,926,914 bp,基因组GC含量为46.53%,共得到3 732个完整的CDS,占整个基因组序列的88.62%. 非编码RNA中包含27个rRNA和86个tRNA,分别占整个基因组序列的1.05%和0.17%(图 2),且该菌中未发现质粒. 将该菌的基因组测序结果提交GenBank,登录号为CP077672.
-
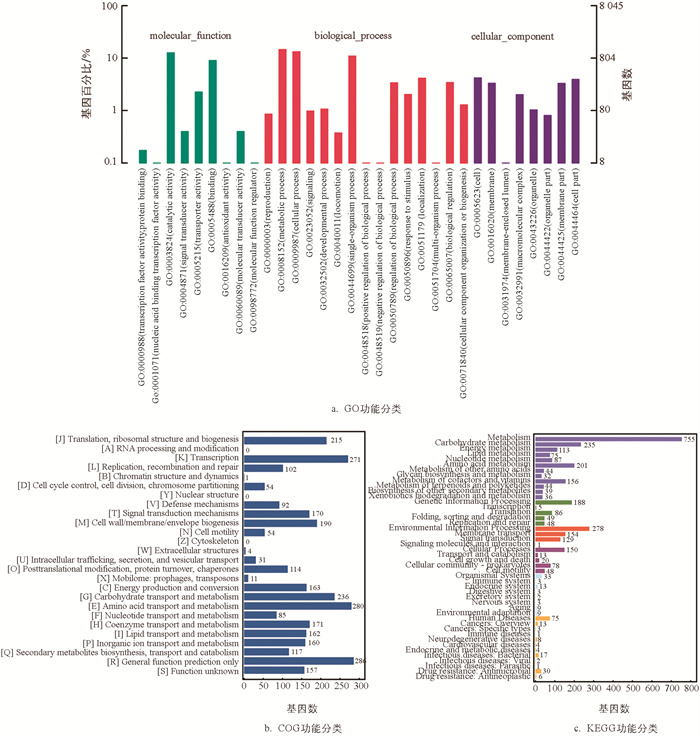
对B. velezensis SWUJ1菌株编码基因在GO数据库进行功能注释分析(图 3a),结果表明在分子功能中参与催化、结合、运输活性相关的基因占比较大;生物学过程中参与代谢过程、细胞过程和单组织过程类别的基因较多;细胞组分中参与细胞、细胞膜、细胞部位、细胞膜部位的基因比例较大,表明B. velezensis SWUJ1的基因多集中在酶催化、代谢以及细胞分化等过程. 将CDS与COG数据库比对分析,结果表明B. velezensis SWUJ1菌株基因组中编码氨基酸转运和代谢蛋白、转录蛋白和碳水化合物转运代谢蛋白的比例较大(图 3b). 对B. velezensis SWUJ1的基因组编码蛋白进行KEGG功能分类统计,结果表明该菌参与代谢功能的基因占比最多,其中编码碳水化合物转运与代谢基因的数目占比达22.86%(图 3c).
基因功能注释结果显示,B. velezensis SWUJ1基因组中含有属于NRPS途径的表面活性素、丰原素、溶杆菌素和嗜铁素4类抑菌物质合成相关基因,以及由PKS-NRPS途径催化的伊枯草菌素(Iturin)和聚酮类化合物Bacillaene合成相关基因(表 2). 其中comA可激活由srfAA,srfAB,srfAC构成的操纵子,从而调控表面活性素的合成[26];fenC,fenD,fenE,fenA和fenB为编码丰原素合成酶的基因[27];bacA,bacG,bacF的编码蛋白是成熟溶杆菌素形成的关键酶[28];编码双模块非核糖体肽合成酶的基因dhbF参与嗜铁素的合成[29]. 与表面活性素、丰原素不同,伊枯草菌素由PKS-NRPS杂化复合物合成,ituA,ituB和ituC是其操纵子的3个开放阅读框[30];聚酮类化合物Bacillaene也是杂化PKS-NRPS的自然产物,pksJ,pksL和pksM等参与了Bacillaene的生物合成[31].
-
通过antiSMASH 5.0.0软件在线预测B. velezensis SWUJ1菌株的次级代谢产物,结果显示该菌基因组中存在13个次级代谢产物合成基因簇. 其中,除萜烯(Terpene)、第3类聚酮类化合物(T3PKS)、细菌素(Bacteriocin)和Cluster 11代谢物类型未知外,其余8个基因簇均可以合成芽孢杆菌中常见且具抑菌活性的次级代谢产物:Cluster 1,表面活性素合成基因簇;Cluster 2,丁酰苷菌素合成基因簇;Cluster 4,大环内酯合成基因簇;Cluster 5,Bacillaene合成基因簇;Cluster 6,丰原素合成基因簇;Cluster 10,Difficidin合成基因簇;Cluster 12,铁载体合成基因簇;Cluster 13,溶杆菌素合成基因簇. Cluster 1,Cluster 6,Cluster 11和Cluster 12基因簇均通过非核糖体途径合成相应的次级代谢产物(表 3).
-
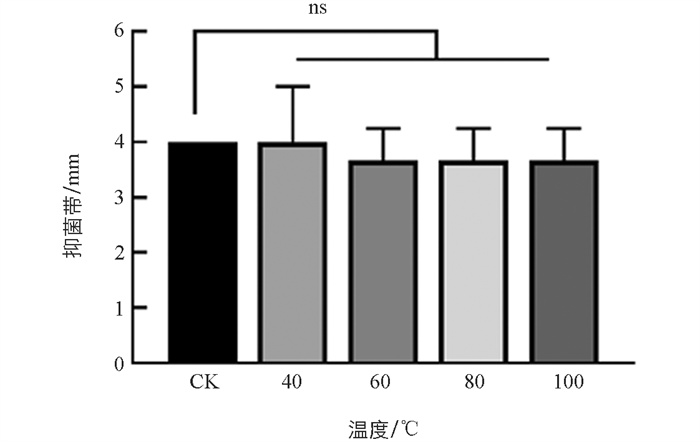
B. velezensis SWUJ1菌株发酵滤液经不同温度热处理后,对病原菌的抑菌活性与未经热处理的对照相比差异无统计学意义,其中40 ℃热处理30 min后抑菌能力无明显变化,60,80,100 ℃热处理30 min后抑菌能力略有下降(图 4)(p>0.05),表明发酵上清液中的活性物质具有较好的热稳定性,有利于后续B. velezensis SWUJ1菌株生防制剂的制备和应用. 综合B. velezensis SWUJ1菌株全基因组分析及活性发酵液的热稳定性结果,初步判断抑菌物质可能为脂肽类物质或小分子活性物质,有利于后续活性物质分离纯化的研究.
-
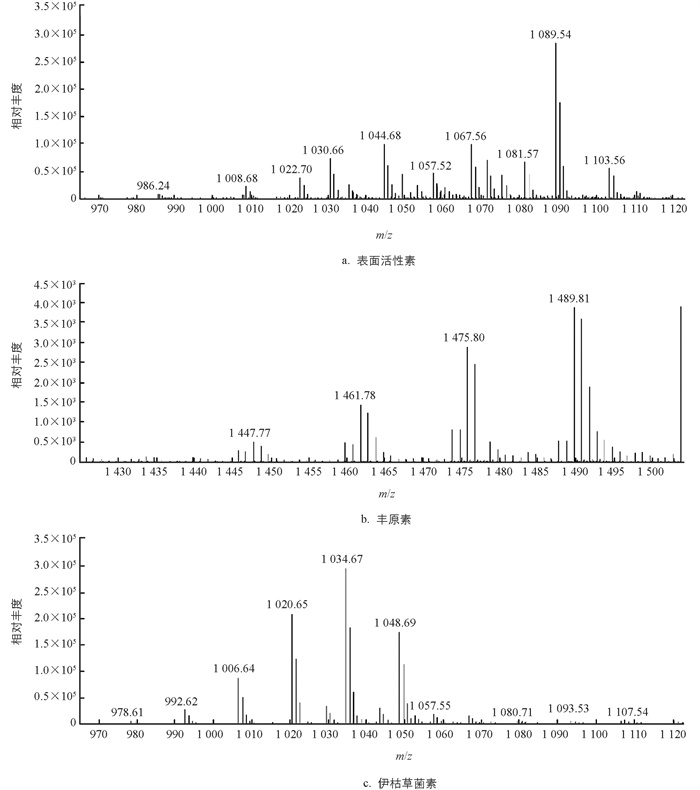
对B. velezensis SWUJ1菌株发酵液粗提物进行LC-MS分析,结果显示该菌株抑菌活性物的粗提物在m/z值为1 008.68和1 022.70处有离子峰(簇)出现,这2个离子峰的相对分子质量依次相差14(脂肪酸链结构“—CH2—”),分别对应于表面活性素(C12—C15)的相对分子质量(图 5a);在m/z值为1 447.77,1 461.78,1 475.80和1 489.81处有4离子峰(簇)出现,分别对应丰原素(C14—C18)的相对分子质量(图 5b);在m/z值为1 006.64,1 020.65,1 034.67和1 048.69处有4离子峰(簇)出现,分别对应伊枯草菌素(C12—C17)的相对分子质量(图 5c). 通过比较离子响应强度发现,抑菌活性物中的丰原素含量较少,表面活性素和伊枯草菌素相对较多. 以上结果表明,菌株B. velezensis SWUJ1可能通过产生脂肽类化合物表面活性素、丰原素和伊枯草菌素抑制病原菌生长.
-
通过纸片抑菌圈法检测菌株发酵液粗提物的抑菌活性,结果表明菌株发酵液粗提物具有较好的抑菌活性(图 6a). 对发酵粗提物进行中压反相柱层析,其中20%甲醇洗脱组分具有良好抑菌活性,进而利用HPLC对该组分进行分离纯化,共收集获得8个组分(Ⅰ-Ⅷ),其中4个组分有抑菌活性(Ⅱ-Ⅴ). 利用Sephadex LH-20凝胶层析对活性最强的Ⅴ组分(保留时间为20.5 min)进一步分离纯化,获得Ⅴ1-Ⅴ4等4个组分. 利用96孔板对各组分的抑菌活性进行检测,结果表明Ⅴ2组分有明显抑菌效果(图 6b).
利用HPLC对Ⅴ2组分进一步制纯,得到单峰物质,命名为Ⅴ21(图 7a). 干燥后为黄色粉末,将其配置成初始质量浓度为5 000 μg/mL的溶液,梯度稀释后检测其对病原菌的抑制作用,结果表明Ⅴ21组分有明显的抑菌作用(图 7b),且其最低抑菌质量浓度(MIC)为200 μg/mL;镜检结果表明,与未加Ⅴ21的病原菌丝相比,质量浓度为150 μg/mL的Ⅴ21组分可使病原菌的部分菌丝扭曲、畸化和膨大(图 7c).
2.1. B. velezensis SWUJ1菌株的抑菌效果
2.2. 拮抗菌株B. velezensis SWUJ1全基因组分析
2.2.1. B. velezensis SWUJ1全基因组组装
2.2.2. 基因预测与功能注释
2.2.3. 次级代谢基因簇分析
2.3. 拮抗菌株B. velezensis SWUJ1发酵滤液热稳定性
2.4. 拮抗菌株B. velezensis SWUJ1发酵液脂肽类抑菌物质的分离鉴定结果
2.5. 拮抗菌株B. velezensis SWUJ1发酵液小分子抑菌物质的分离纯化结果
-
分离自土壤环境中的B. velezensis SWUJ1菌株对香樟炭疽病菌具有较好的抑制作用,且通过对培养基成分和发酵条件进行优化,显著提升了B. velezensis SWUJ1的抑菌活性[20],但有关该菌株的抑菌活性物质及抑菌机制尚不清楚. 本研究对B. velezensis SWUJ1菌株进行了全基因组测序,并对抑菌活性物质进行分离纯化,以期初步探究该菌的抑菌机制. 结果表明B. velezensis SWUJ1菌株具有合成表面活性素、丰原素、Bacillaene和Bacilysin等抗菌物质的相关基因簇,通过LC-MS分析进一步发现该菌发酵液中存在表面活性素、丰原素和伊枯草菌素3种脂肽类抑菌物质,并获得了一个具有较好抑菌活性的Ⅴ21组分,其最小抑菌浓度为200 μg/mL. 徐杨等[32]从海洋枯草芽孢杆菌3512A获得的脂肽类抗菌化合物在氘代试剂中溶解性差,未能获得理想的二维核磁共振数据,利用电喷雾飞行时间质谱解析也未能获得氨基酸裂解顺序,表明获得的抗菌化合物可能是新型脂肽类成分. 本研究获得的Ⅴ21组分在氘代试剂中溶解性亦不佳,仅由一维核磁共振氢谱、碳谱图谱分析尚不能得到其确切结构,经微谱数据比对,可能是Bacillopeptins或Bacillomycin D等脂肽类物质,其结构需进一步确定. 黄承敏等[33]研究表明芽孢杆菌HN-7菌株发酵上清液经121 ℃处理20 min后仍具有很强的抑菌活性. 本研究中B. velezensis SWUJ1菌株的抑菌活性发酵滤液亦具有较好的热稳定性,其在100 ℃处理后抑菌活性无显著变化,说明B. velezensis的活性滤液具有较高的热稳定性,这将有利于其在田间施用.
B. velezensis是一种十分具有生物防治潜力的优势菌种. 刘雪娇等[34]在B. velezensis 3A3-15脂肽类抗生素的粗提物中发现了表面活性素(C14—C15),该菌产生的抑菌物质可使尖孢镰刀菌菌丝发生膨大、弯曲、缠绕等畸化现象. 迟惠荣等[35]利用基质辅助激光解吸电离飞行时间质谱在B. velezensis V4中检测到了表面活性素和伊枯草菌素等抑菌物质. Li等[36]发现B. velezensis 1B-23不仅可以产生表面活性素、伊枯草菌素,也可产生大环内酯、环二肽等抑菌物质抑制多种病原菌. 后续研究将在本文研究基础上,进一步挖掘其生防相关基因,展开关键遗传调控元件及表达系统的研究,深入探究该菌拮抗病原真菌的抑制机理.



 下载:
下载: