-
开放科学(资源服务)标志码(OSID):

-
百合是百合科(Liliaceae)百合属(Lilium)多年生草本球根植物,是世界上具有重要经济价值的观赏植物之一,可用作鲜切花、盆栽花卉和花园造景,一些百合品种也作为食用球茎和药源植物被广泛种植[1-2]. 百合生长过程中易受到多种病害的威胁,常发生真菌性病害如灰霉病、立枯病、炭疽病、斑点病、疫病以及病毒病等,给百合生产带来巨大的经济损失[3-5].
在病原体攻击下,植物已经进化出不同的防御机制以便在逆境中生存,除了植物已形成的有效结构和化学屏障的组成型防御机制外,植物还具有诱导型防御机制,在病原体攻击时被诱导. 诱导型防御机制包括细胞壁交联、过敏性反应、活性氧(ROS)的产生和次生代谢物的积累,以及病程相关蛋白(Pathogenesis-related Proteins,PRP或PRs)的产生[6-8]. 病程相关蛋白是研究较为深入的植物防御蛋白之一,是植物防御系统的核心组成部分[9-10]. 根据其蛋白质序列的相似性、酶活性和其他生物学特征将PR蛋白分为17个家族[11];其中PR10家族是该家族的重要成员,具有抗真菌、抗细菌和抗病毒等活性[12-14]. 迄今为止,已经从70多种植物中分离出100多个PR10蛋白和PR10基因序列[15]. 在玉米中研究发现PR10蛋白可作为抵抗不同真菌和病原菌的抗菌剂,并可应用于生产抗真菌药物或通过转基因获得抗真菌植株[12]. 在欧洲李感染褐腐病期间发现其PR10蛋白的转录本水平升高,并分析发现PR10可能与真菌病害抗性有关[16]. 在芭蕉中,PR10基因的编码蛋白均具有β-1,3-葡聚糖酶和核糖核酸酶活性,并能抑制烟曲霉的生长[15].
为研究百合“索邦”PR10基因的生物学功能,本研究在研究室已构建的灰霉菌(Botrytis elliptica)接种后不同时间百合“索邦”叶片的转录组数据库[17]中筛选获得受病菌侵染后显著诱导表达的病程相关蛋白基因LhSorPR10-2,并克隆得到该基因的全长序列. 通过实时定量PCR分析LhSorPR10-2在百合“索邦”不同组织中的表达模式,以及病原菌处理、激素处理和高低温处理后该基因的诱导表达情况,并对其启动子的顺式作用元件进行分析. 本研究旨在了解百合PR10基因的有关特性及在抗病防御反应中的作用,为今后揭示该基因的抗性分子机制提供理论基础.
全文HTML
-
基因克隆、组织表达特异性分析及灰霉菌(Botrytis elliptica)侵染分析所使用的植物材料为种植于西南大学花卉实验室温室的1龄东方百合“索邦”植株. 培养条件为:光照强度20 000 lx,光周期为光照16 h和黑暗8 h,昼25 ℃/夜20 ℃,湿度为85%.
尖孢镰刀菌处理、激素处理和温度胁迫分析表达的植物材料均为百合“索邦”组培苗. 培养基为:MS+TDZ 0.01 mg/L+NAA 0.2 mg/L,培养条件同上.
-
根据百合“索邦”感染灰霉病的转录组数据库筛选出的基因序列设计LhSorPR10-2特异引物,以百合叶片cDNA为模板,使用Primer Star Max酶进行PCR扩增,反应条件为98 ℃ 10 s,53 ℃ 5 s,72 ℃ 4 s,31个循环. 在开放阅读框设计引物LhSorPR10-2-DNA,以百合“索邦”叶片基因组DNA为模板,使用HiFi酶进行扩增,反应条件为94 ℃ 5 min,94 ℃ 30 s,53 ℃ 30 s,72 ℃ 30 s,72 ℃ 10 min,29个循环(表 1).
利用LhSorPR10-2的蛋白序列作为查询序列,NCBI数据库进行Blastp搜索,结果显示与麝香百合的LlPR107(AAD17336.1)[18]基因相似度高达95%. 因此,以麝香百合的LlPR107为百合“索邦”的LhSorPR10-2同源基因,根据麝香百合LlPR107的启动子序列(KC815691.1)[19]设计引物LhSorPR10-2-pro,并以百合“索邦”叶片DNA为模板,使用Top taq酶进行PCR扩增,反应条件为94 ℃ 5 min,94 ℃ 30 s,57 ℃ 30 s,72 ℃ 1 min 20 s,72 ℃ 10 min,30个循环(表 1).
-
通过MegAlign软件将测序结果与预测序列进行比对,最终得到目的基因序列,将得到的目的基因序列在NCBI上进行BLAST分析,同时进行生物信息学分析. 所用软件及网址如表 2.
-
分别采取1龄盛花期正常生长的百合“索邦”植株的茎、叶、花瓣、雌蕊、花药、花丝、鳞片及根为组织表达特异性分析的样品,于液氮中冷冻后,-80 ℃保存以提取RNA. 用打孔器取灰霉菌菌饼接种于1龄百合叶片,以清水为对照,取接种后3 h,6 h,12 h,24 h,36 h,48 h的叶片染病部位为样品,每个样品取3个生物重复,进行病原菌侵染后的基因表达分析.
选取苗高5 cm,球茎0.5 cm的百合“索邦”组培苗放置于无菌水中预培养24 h后进行尖孢镰刀菌及不同激素处理. 尖孢镰刀菌处理:预处理后的组培苗放入106 cfu/mL尖孢镰刀菌悬浮液中30 min后,转入无菌水中培养,以无菌水处理为对照,取侵染后3 h,6 h,12 h,24 h,36 h和48 h的百合组培苗根部作为样品. 不同激素处理:预处理后的组培苗分别移至100 μmol/L MeJA,200 μmol/L SA和1mmol/L ETH的激素溶液中,放置30 min,再转入无菌水中培养,取处理后0 h,1 h,3 h,6 h,12 h,24 h和48 h的组培苗根部作为样品. 温度胁迫处理:将预培养了两天的“索邦”组培苗分别放置于0 ℃和50 ℃恒温恒湿培养箱,湿度均为85%,取处理后0 h,3 h,6 h,12 h,24 h和48 h的组培苗叶片为样品. 以上处理均是每个样品取3个生物重复,液氮速冻后,放置于-80 ℃冰箱中,保存备用以提取RNA.
荧光定量PCR以LhSorActin,LhSor18s作为内参基因进行表达量分析,反应条件:95 ℃ 30 s;95 ℃ 5 s,58 ℃ 5 s;40个循环. 结果通过Bio Rid CFX软件剔除误差较大数据后,导出数据至GraphPad Prism 8.0.2进行数据分析并作图,使用SPSS 20.0软件进行差异统计学分析,采用Dunnnett检验,p<0.05时表示差异有统计学意义.
1.1. 试验材料
1.2. LhSorPR10-2及其启动子序列克隆
1.3. LhSorPR10-2生物信息分析
1.4. qRT-PCR的植物材料及数据处理
-
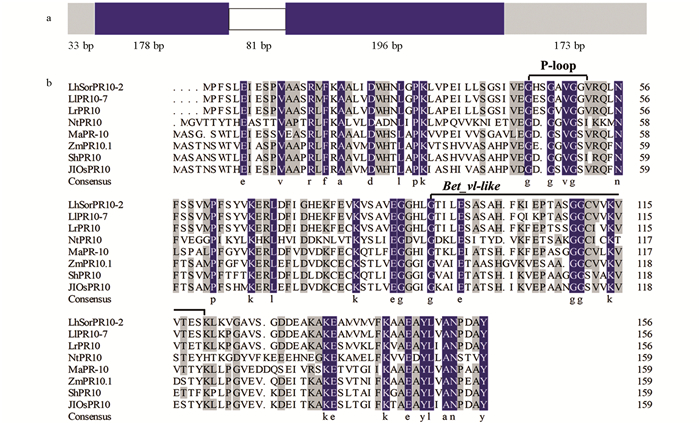
以百合“索邦”叶片的cDNA为模板,RT-PCR扩增获得680 bp的LhSorPR10-2基因序列,包含474 bp的开放阅读框,编码157个氨基酸,5′UTR和3′UTR的长度分别为33 bp和173 bp. 此外,以百合“索邦”叶片的基因组DNA为模板进行PCR扩增,将LhSorPR10-2的cDNA与DNA序列进行比对,发现含有81 bp的内含子(图 1a). LhSorPR10-2蛋白分子量为16.6 KD,理论PI值为5.72,为稳定蛋白和疏水性蛋白,并且不具有跨膜结构和信号肽,对LhSorPR10-2进行亚细胞定位预测发现其定位在细胞质中.
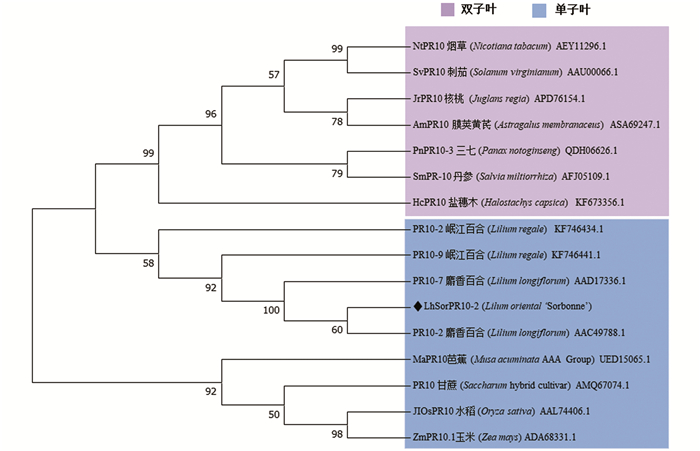
使用DNAMAN对LhSorPR10-2蛋白的氨基酸序列进行多序列比对(图 1b),发现其具有PR10蛋白保守结构域P-loop和Bet_v1-like. 与麝香百合(AAD17336.1)、岷江百合(KF746434.1)、芭蕉(UED15065.1)、甘蔗(AMQ67074.1)、玉米(ADA68331.1)和水稻(AAL74406.1)这6个单子叶植物的PR10蛋白相似性较高,分别为94.2%,88.3%,57.8%,53.2%,53.2%和53.2%(图 1b). 利用MEGA7.0构建LhSorPR10-2蛋白系统进化树,结果如图 2,LhSorPR10-2与麝香百合LlPR107关系最近,与麝香百合(Lilium longiflorum)和岷江百合(Lilium regale)的PR10家族成员聚在一起,并与其他单子叶植物如水稻(Oryza sativa)、玉米(Zea mays)等PR10同源蛋白聚类在一支.
-
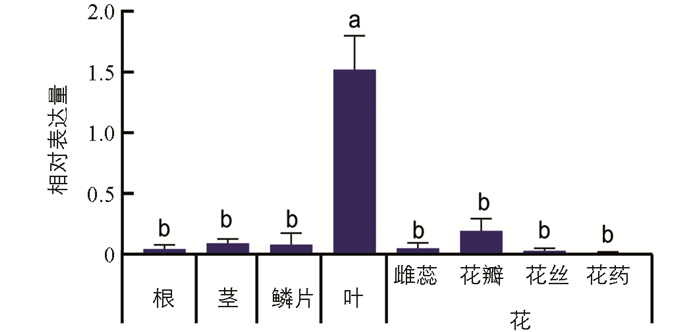
设计基因表达检测特异引物,采用实时荧光定量PCR来检测基因的表达特性. 结果发现LhSorPR10-2基因在百合“索邦”不同组织中表达的差异有统计学意义,主要在叶和花瓣中有表达,并在叶中表达量最高,花瓣次之,在叶片中的基因表达量为花瓣表达量的8倍左右. 而在其余的组织中基因表达量均较低,在花药中几乎不表达(图 3).
-
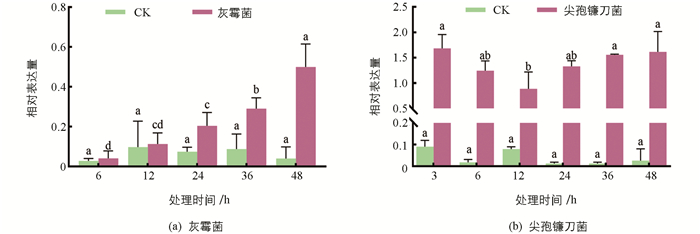
百合“索邦”叶片在灰霉菌和尖孢镰刀菌侵染后,叶片中LhSorPR10-2基因的相对表达量均显著升高,表达量始终高于对照且差异有统计学意义. 在接种灰霉菌后,LhSorPR10-2基因表达量呈现逐渐升高的趋势,6 h,12 h,36 h和48 h时分别为对照的1.6倍、0.9倍、2.7倍、2.9倍和9.5倍,表明灰霉菌能诱导该基因的表达. 在接种尖孢镰刀菌后,LhSorPR10-2基因表达量呈现先升高后降低再升高的趋势,而在对照中几乎不表达,表明尖孢镰刀菌显著诱导了LhSorPR10-2基因的表达(图 4). 综上,灰霉菌和尖孢镰刀菌均能显著诱导LhSorPR10-2基因的表达,LhSorPR10-2可能参与了百合的抗病过程.
-
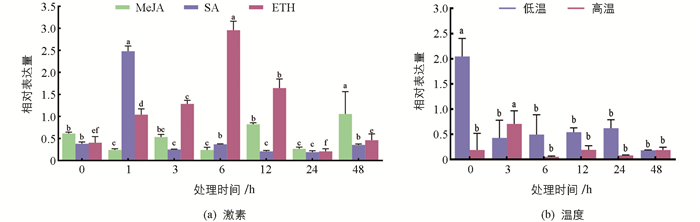
在MeJA,SA和ETH激素诱导下,LhSorPR10-2基因的表达量情况各不相同(图 5). MeJA处理后,LhSorPR10-2表达量变化不大,在48 h达到最高,6 h达到最低;SA处理后,LhSorPR10-2表达量呈现先升高后降低的趋势,并在1 h表达量最高;ETH处理后,LhSorPR10-2表达量也呈现为先增高后降低的表达模式,LhSorPR10-2在6 h表达量最高. 结果表明LhSorPR10-2基因表达受激素诱导表达,但是不同激素的应答机制可能不同. 分别检测高温和低温胁迫对LhSorPR10-2基因表达的影响,结果发现,高温处理下的LhSorPR10-2基因表达量较低,表现为先升高后降低的表达趋势,在3 h时表达量达到最高,48 h表达量最低. 低温处理后LhSorPR10-2的表达量逐渐降低. 结果表明低温和高温胁迫均能一定程度上影响LhSorPR10-2基因的表达(图 5).
-
通过PlantCARE对LhSorPR10-2启动子序列进行顺式调控元件分析,发现该序列含有的元件有15个,除了包含转录必备元件TATA-Box和CAAT-Box之外,还含有5种光响应元件(AE-Box,GATA-motif,GT1-motif,TCT-motif和Sp1). 无氧诱导所必需的顺式作用调节元件ARE,参与胚乳表达的顺式调控元件GCN4_motif,赤霉素反应元件P-box,参与蛋白代谢调节的顺式作用元件O2-site,MYC特异识别位点和MYB识别位点(表 3). 这些顺式作用元件表明LhSorPR10-2基因可能响应光信号、植物激素和逆境胁迫.
2.1. LhSorPR10-2的克隆和生物信息分析
2.2. 百合LhSorPR10-2基因表达模式分析
2.2.1. LhSorPR10-2组织表达特性分析
2.2.2. LhSorPR10-2基因的生物胁迫诱导特性分析
2.2.3. LhSorPR10-2基因的非生物胁迫诱导特性分析
2.3. LhSorPR10-2基因启动子顺式作用元件分析
-
本研究从百合“索邦”中分离并克隆获得LhSorPR10-2基因,全长761 bp,开放阅读框为474 bp,编码158个氨基酸,等电点为5.72,含有一个内含子,这与华东葡萄、岷江百合和月季等克隆获得的PR10同源基因结果相似[20-22]. 生物信息学分析发现LhSorPR10-2蛋白分子量为16.6 KD,理论PI值为5.72,为稳定蛋白和疏水性蛋白,不具跨膜结构和信号肽,并且存在于在细胞质内,这与大多数研究PR10蛋白家族的序列和结构特征结果一致[13, 23]. 系统进化分析发现LhSorPR10-2与麝香百合LlPR107关系最近,并与麝香百合和岷江百合的PR10家族成员聚集在一起,推测其可能具有相似的功能.
PR蛋白广泛分布于植物所有器官,在叶中特别丰富,并占叶片中总蛋白质的5%~10%[11]. 本研究中组织表达特性分析发现LhSorPR10-2基因主要在叶和花瓣中有表达,并在叶中表达量最高,这与已有的研究结果一致. 植物在自然环境中不可避免地会受到各种生物及非生物的胁迫,而PR10蛋白对于植物防御具有重要作用[24]. 番红花CsPR10具有抗尖孢镰刀菌活性,通过激活茉莉酸途径,从而参与防御反应[25]. 辣椒真菌抗性品种和易感品种在真菌感染后,只有具真菌抗性的辣椒中有bacPR10基因表达量的积累,表明该基因在逆境防御中可能具有重要功能[26]. 菠菜SoPR10的表达受硝酸盐胁迫和其他非生物胁迫包括聚乙二醇、NaCl、水杨酸和H2O2的诱导,表明其可能在硝酸盐胁迫和其他逆境下发挥重要作用[24].
茉莉酸甲酯(MeJA)、水杨酸(SA)和乙烯利(ETH)等植物激素被描述为植物胁迫中的主要信号分子,其信号通路已被广泛研究[27-28]. 植物受到病原菌侵染后,防御信号通路如水杨酸(SA)和茉莉酸(JA)被激活,进一步导致PR蛋白的积累,从而最大限度地减少病原体扩增和未感染植物器官中的病原菌感染[11]. 本研究中,百合“索邦”叶片在接种灰霉菌和尖孢镰刀菌后,LhSorPR10-2基因的相对表达量均明显诱导升高,且表达量始终高于对照且差异有统计学意义,说明LhSorPR10-2可能参与百合抗真菌的过程. 在MeJA,SA和ETH等外源激素以及高温(50 ℃)和低温(0 ℃)胁迫处理下,LhSorPR10-2转录水平的表达量表现出不同程度的响应,说明该基因在不同激素处理及温度胁迫中的应答机制可能有所不同. 此外,LhSorPR10-2启动子区有与逆境胁迫相关的顺式作用元件,推测其可能在生物及非生物胁迫过程中发挥作用.
本研究在百合“索邦”叶片转录组数据库中筛选获得受灰霉病菌侵染后显著诱导表达的病程相关蛋白基因LhSorPR10-2,并克隆得到该基因的全长序列. 通过实时定量PCR分析了LhSorPR10-2在百合“索邦”不同组织中的表达模式,以及病原菌处理、不同激素处理、高温及低温胁迫处理后的诱导表达情况,并对其启动子的顺式作用元件进行了分析. 然而,LhSorPR10-2基因在百合“索邦”的具体抗病作用以及受灰霉菌诱导后激活了哪些下游基因? LhSorPR10-2如何在百合-灰霉病互作过程中起作用等问题仍有待进一步研究.



 下载:
下载: