-
开放科学(资源服务)标识码(OSID):

全文HTML
-
纳米酶基于材料在纳米尺度展现出与其宏观尺度不同的新特性. 2004年,Manea等[1]最先提出了“纳米酶”,应用官能团功能化的金纳米粒子作为催化剂,模拟核糖核酸酶剪切磷酸二酯键的催化活性,但其催化活性主要来自其表面修饰的官能团,而不是其本身的特性. 起初,纳米材料被认为是化学惰性物质. 2007年阎锡蕴院士团队在研究如何用纳米材料进行抗原检测时发现了一个“诡异”现象:Fe3O4纳米颗粒在人体组织中可以吸附带电荷的抗原分子,并且可以使抗原变色. 而使抗原变色原本需要加入辣根过氧化物酶才会发生,经过多次实验发现Fe3O4纳米颗粒与辣根过氧化物酶具有类似的性质. 随即首次报道了Fe3O4磁性纳米粒子的本征过氧化物酶活性[2]. 在随后的十多年内,大量关于纳米酶的研究不断涌现. 如贵金属纳米材料、半导体纳米材料、碳纳米材料和金属有机框架材料等. 近年来,纳米酶独特的理化特性也成为研究者关注的热点,例如可调节带隙宽度、独特的吸收光谱和局部表面等离子体共振等. 研究者将纳米酶的理化性质与其类酶活性相结合,制备了一系列具有优越性能的纳米酶,并增强纳米酶的应用功能. 例如,通过利用纳米材料的光热性质来提升纳米酶的催化活性,可以形成光增强型的催化体系. 此外,利用光、声、电磁和辐射等刺激激活纳米材料的催化特性也可以提高催化效率[3]. 因此,纳米酶理化性能的结合将扩大其“内涵”,为改善纳米酶催化特性及扩大生物应用范围提供新思路. 本文总结了纳米酶的合成方法、分类、催化原理和生物医学应用.
-
纳米酶的制备方法有很多种,主要来自一些金属、金属氧化物、非金属氧化物以及有机金属框架纳米材料及上述纳米材料复合体系的制备,如图 1所示.
-
贵金属纳米酶主要指过渡元素中的贵金属元素金、银、铂、钯和铱等金属纳米材料或它们的合金纳米材料,贵金属纳米酶中最常用的是金纳米颗粒,继金纳米颗粒被报道具有类葡萄糖氧化酶性质后,其在生物检测及疾病诊断方面应用广泛,金纳米颗粒的制备方法常用的有柠檬酸盐还原法和Brust-Schiffrin法.
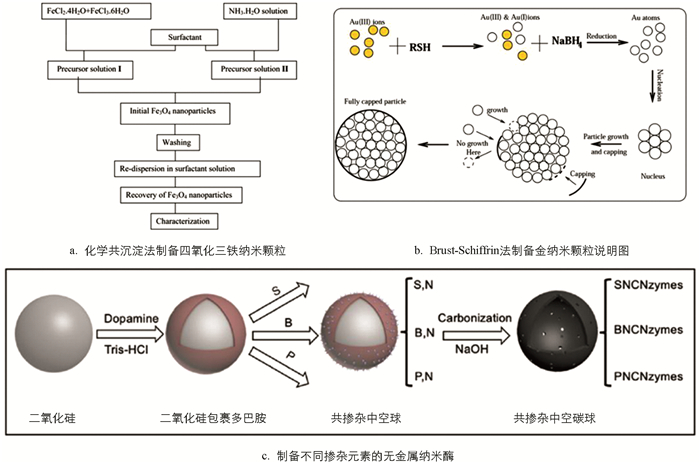
金纳米颗粒制备比较经典的方法是柠檬酸盐还原法,即Turkevich-Frens法,该方法是应用最广的制备尺寸均一的金纳米颗粒方法,后面发展出的很多方法也是在此基础上的延续. 在接近沸点(100 ℃)的水溶液中用柠檬酸钠还原金前体. 这个过程产生稳定的金纳米颗粒水溶液(金胶体或金溶胶),原因是柠檬酸根离子既作为还原剂又作为保护剂. 这种制备方法在生物方面应用较广. 因为该方法制备的金纳米颗粒更利于表面修饰官能团. 后来Hanžić等[4]也报道了用γ射线辐射氯金酸/柠檬酸盐溶液前体,通过改变金与柠檬酸盐的摩尔比可以得到不同尺寸和不同特征吸收的金纳米颗粒;Brust-Schiffrin法利于在室温下合成粒径较小(2~2.5 nm)[5],同时具有高度稳定性的功能化金纳米粒子. 该方法极大地影响了后续金纳米颗粒制备方法的发展,其他很多贵金属纳米材料也是参考该方法合成的. 在烷硫醇和二烷基二硫化物的硫醇配体作用下,加入硼氢化钠还原三价的金粒子,再生长成金纳米粒子.
一种金属纳米材料可能会有多种酶活性,例如铂纳米粒子具有4种类酶活性,包括超氧化物歧化酶、过氧化氢酶、氧化酶和过氧化物酶. 其中一些活性会相互干扰. 例如,作为抗氧化酶模拟物,它们的超氧化物歧化酶和过氧化氢酶活性对清除活性氧(ROS)非常有帮助. 然而它们的氧化酶或过氧化物酶活性可能同时氧化一些天然抗氧化剂,从而影响最终的抗氧化功效. 因此,微调不同的酶活性对于优化其功能非常重要. 一些研究工作者将其做成多种金属合金,通过改变电子结构来微调某种类酶活性[6].
-
二氧化铈和Fe3O4是常用的金属氧化物纳米酶. 氧化铈纳米粒子以混合价态(Ce3+,Ce4+)存在,具有许多独特的性质,已被证明在生物医学和催化应用中具有很高的实用性. 据报道,氧化铈纳米粒子具有多酶活性,如超氧化物歧化酶、过氧化氢酶、氧化酶和磷酸酶活性. Roggenbuck等[7]用Ce(NO3)3浸渍介孔CMK-3碳,制备介孔CeO2.
由于铁氧化物纳米酶巨大的潜在应用前景,近年来此领域的研究较多,例如磁性药物靶向、磁共振成像以及在肿瘤微环境响应型治疗等. Fe3O4的制备方法众多,例如电弧放电、机械研磨、激光烧蚀、微乳化以及有机前体的高温分解等,但是用于生物领域需要其具有良好的分散性以及生物相容性. 作为一种方便且廉价的方法,化学共沉淀法能满足直接制备分散性良好的Fe3O4纳米颗粒的需求. 适当的表面活性剂可以很好地控制纳米颗粒的制备,并且可以很好地控制纳米颗粒的尺寸. 此外,如果严格地控制溶液pH值、反应温度、搅拌速率、溶质浓度和表面活性剂浓度等条件,则可以制备所需形状和尺寸的Fe3O4纳米颗粒. 例如:使用氨水溶液进行改性的二价/三价铁盐共沉淀来合成Fe3O4纳米粒子,该粒子可以很好地分散在水溶液中[8].
多金属过渡金属氧化物因其多价金属离子存在而具有良好的催化性能,近年来也得到了特别关注,其多种类酶活性在肿瘤治疗领域有重要的应用前景. 例如Chang等[9]利用硫化铜钼(Cu2MoS4)构建了负载葡萄糖氧化酶(GOx)的中空介孔Cu2MoS4多功能纳米材料,用于结合化学动力疗法、饥饿疗法、光热疗法、免疫疗法协同治疗癌症.
-
随着具有过氧化物酶活性纳米材料的涌现,人们发现一些非金属纳米材料同样具有类酶活性,特别是具有高稳定性和良好生物相容性的非金属纳米酶,为纳米酶应用于癌症治疗带来新的机遇. Liang等[10]在密度泛函理论(DFT)分析的指导下,利用杂原子改变碳催化剂中的电子平衡和活性位点,制备了一系列非金属原子共掺杂碳材料,包括磷和氮双掺杂多孔空心碳球、硫和氮双掺杂多孔空心碳球、硼和氮双掺杂多孔空心碳球. 制备方法为:多巴胺分子(氮的前体)或三磷酸腺苷(磷的前体)或原硼酸(硼的前体)或左旋半胱氨酸(硫的前体)混合下通过硬模板过程自聚合. 24 h后,产物通过离心、洗涤和干燥后获得. 干燥的粉末在氮气氛围内于800 ℃碳化2 h. 然后用氢氧化钠蚀刻、洗涤和干燥后得到不同掺杂的非金属纳米酶.
-
一些有机金属框架也有类似某种天然酶活性的性质,例如Chen等[11]提出MOF-818的活性位点三核铜中心具有类天然儿茶酚氧化酶活性,并且具有良好的特异性. 儿茶酚氧化酶特异性催化邻二酚氧化成相应的邻醌,对黑色素和其他多酚类天然产物的生物合成很重要. MOF-818作为一种新型儿茶酚氧化酶纳米酶,具有良好的特异性和高催化活性.
-
目前发现的纳米酶种类繁多,一种纳米材料可能有多种类酶活性,对这些纳米酶的分类也有多种方式. 深入了解纳米酶可能的催化机理将有助于新型高效纳米酶的开发,以合理调控纳米酶的活性. 另外纳米技术的快速发展拓宽了新型纳米酶的探索途径. 结合模拟和理论计算,这些纳米酶可能的催化机理逐渐被阐明,这些研究可能有助于提高纳米酶的催化活性. 图 2是对纳米酶的分类及机理的简要展示.
-
氧化酶是过氧化物酶体中的主要酶类,氧化酶约占过氧化物酶总量的一半,包括:尿酸氧化酶、D-氨基酸氧化酶、L-氨基酸氧化酶和L-α-羟基酸氧化酶等. 各种氧化酶作用于不同的底物,其共同特征是氧化底物的同时,将氧还原成过氧化氢. 氧化酶中比较常见具有葡萄糖氧化酶活性的金纳米材料,Li等[12]提出金纳米粒子可以在氧气存在的情况下催化葡萄糖氧化生成葡萄糖酸和H2O2. 通过实验表明,其他金属纳米材料对葡萄糖氧化没有表现出明显的催化能力. 随后,他们在2006年通过一系列实验探索,对这一现象给出了合理的解释[13]. 在碱性和产生H2O2的促进下,水合葡萄糖阴离子与金表面原子的相互作用可以形成富电子的金,通过亲核吸附活化分子氧. 双氧中间体的Au+-O2-或Au2+-O22-可以作为将电子从葡萄糖转化到氧气的桥梁.
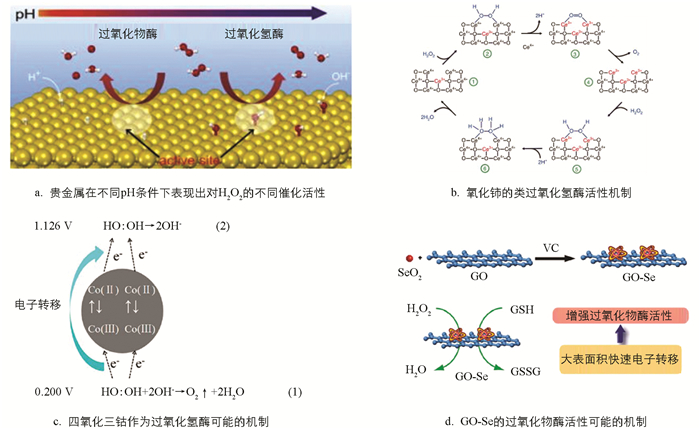
2015年Shen等[14]通过密度泛函理论系统地证明钯、铂、银和金及其合金等贵金属的氧化酶活性机理,即金属表面上吸附的单原子氧原子具有Brønsted-base特征. 它们能够从周围的分子中提取酸性氢作为氧化剂. 预覆盖有氧原子的金具有很强的氧化能力,能够氧化一氧化碳和乙醛. 因此,贵金属的类氧化酶活性机制总结为以下两个反应方程式[15]:
其中,公式(2)中左边的S代表氧化底物. 也就是说,金属催化吸附氧气分子离解产生氧原子,然后氧原子从底物(如TMB和抗坏血酸)中提取氢,完成O2对底物的氧化. 在整个反应中,金属表现出氧化酶活性. 由于自旋守恒,3O2与有机底物的直接反应产生了具有非零磁矩和高能量的化合物,因此在自然界是不稳定的. 当3O2离解成金属上的氧原子时,由于3O2的两个反键π*轨道已经被自旋向上的电子占据,它的键序通过接受金属的自旋向下的电子而降低到零. 自旋向下的电子从金属中转移出来,导致金属上自旋向上的电子过多. 因此,这个过程本质上将磁矩从3O2转移到金属,产生化学吸附的零磁矩氧原子. 根据自旋守恒定律,这个氧原子可以与有机底物反应生成稳定的非磁性化合物. 这定性地解释了贵金属的类氧化酶活性的起源.
还有一些金属氧化物特异性氧化某种底物主要是依靠其高价态金属的氧化性,例如2014年,Ragg等[16]证明了在生理条件下,纳米氧化钼(MoO3)具有一种类亚硫酸盐氧化酶活性,亚硫酸盐氧化酶(SuOx)是一种位于线粒体上的含钼酶,可以催化有毒的亚硫酸盐氧化为硫酸盐,细胞色素c是电子受体. SuOx在细胞内解毒过程中很重要,如果缺乏可能导致一些疾病,而氧化钼这种活性可能是亚硫酸盐氧化酶缺乏症的潜在治疗剂. SO32-首先与MoO3纳米颗粒结合,被氧化成SO42-,Mo从六价还原成四价. 通过两个电子转移步骤,然后被电子受体氧化到初始价态,形成电子供应链. 另外二氧化铈[17]、二氧化锰[18]、铁酸钴[19]、铁酸锰[20]以及上述纳米材料复合物具有氧化酶活性的原理主要是类似上述化合物的氧化性质.
-
辣根过氧化物酶比活性高,分子量小,纯酶容易制备. 广泛分布于植物界,辣根中含量高,它是由无色的酶蛋白和棕色的铁卟啉结合而成的糖蛋白,是临床检验试剂中的常用酶. Liu等[21]发现金纳米颗粒有类过氧化物酶活性,可能的机理是金纳米材料先吸附H2O2分子,然后H2O2的O—O键断裂形成两个羟基自由基. 同时,形成的羟基自由基通过与金表面电子交换而具有稳定性,用于后续的催化作用[22]. 银、铂、钯等贵金属合金的类过氧化物酶活性的催化机理类似. 许多碳基纳米材料同样表现出优异的类过氧化物酶性质. Zhao等[23]以石墨烯量子点(GQDs)为例探索了其催化机理,结合实验数据和理论计算,发现—C=O和—O=CO—基团分别可以作为催化活性位点和与底物结合位点. 这些基团的修饰可以有效提高石墨烯量子点的催化活性. 相反,—C—OH的存在会抑制它们的催化性能.
继2007年阎锡蕴团队发现Fe3O4的类过氧化物酶活性后,大量铁基纳米材料的过氧化物酶活性被发现. Cleland等[24]报道Fe3O4纳米粒子的催化机制可能遵循乒乓反应机制,Fe3O4可以首先与底物H2O2结合形成羟基自由基,然后生成的羟基自由基将从氢供体(如TMB)中捕获一个氢离子.
-
过氧化氢酶存在于红细胞及某些组织内的过氧化体中,它的主要作用就是催化H2O2分解为H2O与O2,使得H2O2不与O2在铁螯合物作用下反应生成有害的羟基自由基. 具有过氧化氢酶活性的贵金属纳米材料有铂、金、银、钯以及上述金属合金材料. Liu等[21]通过计算得出结论:金属(Au、Ag、Pd和Pt)在低pH条件下表现出类过氧化物酶活性,原因是金属表面H2O2的碱样分解,而在高pH条件下则表现为类过氧化氢酶活性,原因是其表面H2O2的酸状分解. 贵金属在不同pH条件下表现出对H2O2的不同催化活性. 除了贵金属催化剂具有过氧化氢酶活性,一些金属氧化物也具有类过氧化氢酶活性. Pirmohamed等[25]证明了纳米氧化铈依赖其氧化还原状态可以作为过氧化氢酶的替代物,高价态的Ce4+是其催化活性的主要原因. Wang等[26]进一步说明了纳米氧化铈的类过氧化氢酶活性机制. Mu等[27]则通过能斯特方程计算反应的活化能得到四氧化三钴可能的过氧化氢酶活性机制,Co3O4纳米材料作为一种半导体材料,可以作为电子传递介质,类似于天然过氧化氢酶.
-
谷胱甘肽过氧化物酶(GPx)是一种细胞内抗氧化酶,可将过氧化氢酶水解为水,以限制其有害作用. 研究者目前发现具有类谷胱甘肽过氧化物酶活性的纳米材料有五氧化二钒、硒以及硒复合物和锰基纳米材料. Huang等[28]发现石墨烯负载硒(GO-Se)纳米复合材料具有出色的类谷胱甘肽过氧化物酶活性,可保护细胞免受氧化应激. 与单独硒纳米粒子相比,GO-Se纳米酶表现出更高的GPx模拟催化效率. 催化反应可能遵循乒乓机制:H2O2与纳米硒反应,得到的氧化硒中间体将与硫醇(如GSH)反应生成GSSG,硒成分将返回到初始纳米硒状态. 在谷胱甘肽还原酶(GR)及其辅酶NADPH存在下,GSSG可以再次还原为GSH,然后纳米硒继续与其他H2O2分子反应.
-
超氧化物歧化酶(SOD)是生物体内存在的一种抗氧化金属酶,它能够催化超氧阴离子自由基歧化生成氧和过氧化氢,在机体氧化与抗氧化平衡中起到至关重要的作用,与很多疾病的发生、发展密不可分. 具有超氧化物歧化酶活性的纳米材料主要有金、铂、钯及其合金等贵金属纳米材料、氧化铈和一些锰基纳米材料. Liu等[21]同样分析了贵金属纳米材料超氧化物歧化酶活性的机理. 超氧阴离子自由基O2·是一种碱性物质,pKb=9.12,它很容易从水中捕获一个质子,形成HO2·和OH-,贵金属的晶面具有较小的活化能,吸附的HO2·基团会在贵金属晶面上重排,产生H2O2和O2. 一些金属氧化物同样具有类超氧化物歧化酶活性,例如氧化铈,由于Ce3+和Ce4+以及氧空位的存在,纳米氧化铈具有优异的催化性能[29].
-
纳米酶的种类还有很多,例如Korschelt等[30]发现缺氧型CeO2-x纳米棒可以作为一种有效的绿色尿酸酶模拟物,在室温条件下催化尿素水解. 其活性比天然的菜豆脲酶低一个数量级. 通过镧取代调整Ce4+/Ce3+的比例,研究了氧化铈的表面性质. 虽然镧的取代增加了表面缺陷的数量,但纳米铈表面Ce4+位置的减少导致催化效率的降低. 虽然天然的刀豆脲酶受到Cu2+离子的强烈抑制,但氧化铈的尿酸酶活性不受Cu2+的影响. Celardo等[31]还发现V2O5纳米线具有加卤过氧化物酶的活性,可以应用于阻止海洋微环境中生物膜的形成. 在H2O2存在下,V2O5纳米线可以催化卤离子,如氯离子和溴离子,产生次卤酸. 生成的次卤酸会对海洋微生物造成氧化损伤,保护船只免受海洋中细菌的黏附. 纳米酶的种类还有很多,在此不再赘述.
-
纳米酶具有优异的物理化学性质和催化活性,在生物医学领域的应用主要有生物传感、疾病治疗、抗菌等方面. 图 3是对纳米酶在生物医学领域应用举例. 纳米酶因其独特类酶催化特性,近年来受到广泛关注,如类过氧化物酶,可以催化底物发生显色反应. 在此基础上,基于纳米酶的新型生物传感器已成功设计并用于检测离子、小分子、核酸、蛋白质等.
-
纳米酶可以用于多种离子检测,如金属离子Cu2+、Ag+、Fe2+等,非金属离子CN-、F-等. 具体如下:Song等[32]2010年利用点击化学,将叠氮化物功能化的磁性二氧化硅纳米粒子点击到乙炔功能化的多壁碳纳米管上制备了复合纳米系统,制备的复合纳米材料比单独的二氧化硅磁性纳米粒子或多壁碳纳米管都具有更高的过氧化物酶样活性. 作为点击化学催化剂,一价铜来源于抗坏血酸钠或谷胱甘肽存在下对二价铜的还原,该纳米复合物的催化活性可用于铜(Cu2+或Cu+)的检测.
Chang等[33]2016年提出了一种简单可行的检测超低浓度银离子的比色法. 牛血清蛋白修饰的金纳米团簇具有类过氧化物酶活性,可以催化H2O2氧化TMB. 一旦引入银离子,银离子选择性地与金发生氧化还原反应,这就导致金纳米粒子的过氧化物酶活性被抑制,在此基础上,建立了银离子比色传感器. Lien等[34]2018年报道了一种简单的一步法合成具有类过氧化物酶活性、分散性良好的氧化石墨烯负载无定形氢氧化钴,并研究了其在检测CN-中的应用. 氰化物离子可以显著抑制石墨烯负载无定形氢氧化钴纳米复合物的催化活性,这使得其可以用于检测水样和实验室废液中的氰化物. 其工作原理是,CN-抑制CoOxH-GO催化H2O2氧化安普尔克斯红(AR)形成荧光间苯二酚. 随着CN-浓度的增加,膜的红色强度降低,这可以用肉眼观察到. 这种高性价比的传感系统可以快速、简单地测定复杂废水样品中CN-的浓度.
-
葡萄糖是一种重要的生物分子,也是一种能量来源和代谢中间体. 人体必须将葡萄糖水平调节在3~8 mmol/L,因为更高的葡萄糖浓度,特别是在糖尿病中,会导致严重的并发症,包括心脏病、肾病、失明等. 因此,使用快速、可靠和准确的传感器来监测体液中的葡萄糖至关重要. Karim等[35]将纳米银包埋在棉织物基质中,用于快速检测复杂生物液体(如尿液)中的葡萄糖含量. 棉织物作为模板的使用不仅提供大量的催化活性位点参与催化反应,棉纤维的吸收特性也有助于在传感过程中快速吸收生物分子,如葡萄糖,从而能够准确检测尿液中的葡萄糖含量.
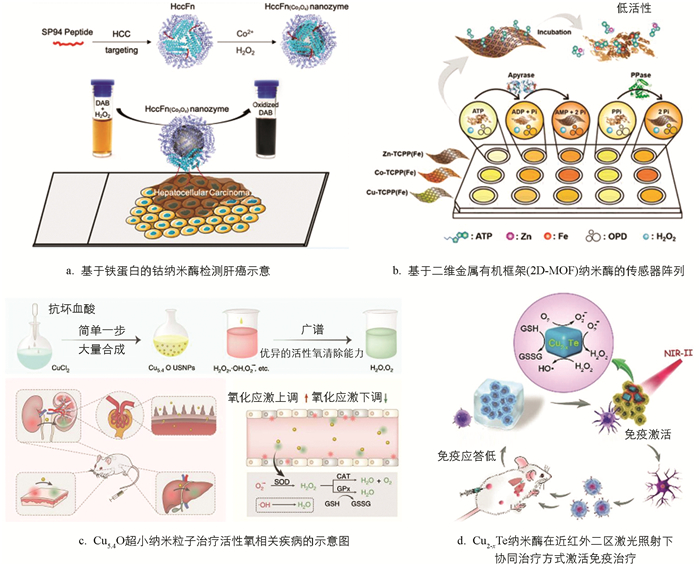
尿素和脲酶在化学和生物化学的发展中起着重要作用. 尿素广泛分布于生物体内,是尿液中的主要含氮成分和蛋白质代谢的终产物,因此尿素的测定在食品工业、环境监测和临床中非常重要. Deng等[36]基于金纳米粒子的类辣根过氧化物酶活性,构建了一个新的检测尿素、脲酶和脲酶抑制剂的传感平台,尿素和脲酶的检测限分别为5 μmol/L和1.8 U/L. Liu等[37]制作了基于二维金属有机框架(2D-MOF)纳米酶的传感器阵列,通过用不同的磷酸盐,包括腺苷酸、腺苷二磷酸、三磷酸腺苷、焦磷酸和磷酸来调节它们的过氧化物酶活性. 传感器阵列不仅成功地用于区分水溶液中的5种磷酸盐,还用于区分生物样品中的5种磷酸盐.
-
单核苷酸的多态性与肿瘤形成和恶化密切相关. 因此,对单核苷酸的检测分析可以为恶性肿瘤的早期诊断和风险评估提供重要参考. 例如Chen等[38]利用金纳米粒子的酶活性和DNA单链分子与DNA双链分子之间的亲和力,报道了一种用于检测DNA的探针. 金纳米粒子通过金硫键对单链DNA有很高的亲和力. DNA修饰在金纳米粒子上会改变金纳米粒子的表面性质,从而限制其类葡萄糖氧化酶活性. 与DNA单链分子相比,双链DNA分子与金纳米粒子的结合能力相对较弱. 在双链DNA的存在下,金纳米粒子仍能保持较高的酶活性来催化葡萄糖的氧化. 在辣根过氧化物酶的帮助下,葡萄糖被氧化的产物H2O2可以与催化氧化相应的底物发生显色反应.
-
酶联免疫吸附试验是辣根过氧化物酶最重要的应用之一. 一些具有类过氧化物酶活性的纳米酶,特别是Fe3O4,可用于抗原、抗体等的免疫检测. 与传统的酶联免疫吸附法相比,这种基于纳米酶的免疫分析方法具有简便、快速、经济、灵敏等优点. 例如He等[39]证明了由金纳米棒负载铂纳米点组成的纳米材料具有类氧化酶、类过氧化物酶和类过氧化氢酶多种活性. 基于上述发现,他们建立了一种基于金包覆铂纳米酶联免疫吸附试验(ELISA)来检测小鼠白细胞介素2(IL-2).
-
纳米酶还应用于多种疾病的诊断. 埃博拉病毒2014年在西非肆虐,迫切需要一种快速、高度灵敏、易于使用的埃博拉诊断检测方法. Demin等[40]用Fe3O4磁性纳米粒子代替胶体金作为纳米酶探针,建立了酶标法检测埃博拉病毒. 该纳米酶探针与埃博拉病毒抗体结合,具有3种功能:识别、分离和条带上埃博拉病毒的可视化. 由于其固有的过氧化物酶活性,纳米酶探针可以催化过氧化物酶底物发生显色反应,从而显著放大信号. 此外,纳米酶探针的磁性为样品中成分的快速分离和富集提供了便捷. 随后Bing等[41]在2019年报道了一种单分散的基于铁蛋白的钴纳米酶,其特异性靶向并可视化临床肝癌细胞(HCC). 钴纳米酶是在肝癌细胞靶向性铁蛋白外壳仿生合成的,通过基因工程方法在铁蛋白表面修饰肝癌细胞特异性多肽SP94. 该纳米酶固有的过氧化物酶活性催化底物发生显色反应,使肝癌肿瘤组织可视化,该研究验证了钴纳米酶具有良好的特异性和敏感性.
-
纳米酶在疾病治疗领域也有诸多应用. 例如,氧化应激与许多急性和慢性炎症疾病有关,但目前临床上可用的治疗方式有限. 开发具有良好活性氧清除能力、生物相容性好的纳米酶是治疗活性氧相关炎症的一种有效的方法. Liu等[42]报道了一种简单高效的一步法制备超小Cu5.4O纳米粒子,该纳米粒子具有多种类酶活性和活性氧清除能力,用于治疗活性氧相关疾病. Cu5.4O纳米粒子同时具有过氧化氢酶、超氧化物歧化酶和谷胱甘肽过氧化物酶特性,在极低剂量下对活性氧介导的细胞损伤具有保护作用,并对改善急性肾损伤、急性肝损伤和促进伤口愈合有很好的治疗效果. 同时,超小尺寸的Cu5.4O纳米粒子能够使纳米材料快速被清除,保证生物安全.
此外,外周β淀粉样蛋白(Aβ)的清除有助于克服血脑屏障(BBB)障碍,消除与阿尔茨海默病(AD)相关的脑源性Aβ. 即便如此,当前开发的清除外周Aβ的治疗方法仍面临着如何避免某些生物分子的干扰,以及如何防止触发免疫反应和凝血的挑战. 近些年,Ma等[43]报道了一种具有增强蛋白质吸附抗性、最小化免疫原性和增强生物相容性的纳米酶(CuxO@EM-K). 该CuxO@EM-K由修饰由3xTg-AD小鼠红细胞膜包裹CuxO纳米酶制成,该红细胞膜具有Aβ靶向性五肽KLVFF. KLVFF作为Aβ特异性配体,与红细胞膜一起选择性地捕获血液中的Aβ. 同时,红细胞膜涂层阻止蛋白质冠状物的形成,从而保持CuxO@EM-K在生物液体中保存的Aβ靶向能力. 更重要的是,具有多种抗氧化酶样活性的CuxO核心稳定了外层红细胞膜,同时减轻了Aβ诱导的膜氧化损伤,从而延长必需的全身循环吸附Aβ.
Ma等[44]制备了铁金属有机骨架纳米颗粒(MIL-101(Fe) NPs)作为纳米酶,通过催化肿瘤微环境中的内源性物质产生活性氧(ROS),诱导癌细胞死亡. 在微波照射下,大量刺激反应性羟基自由基(·OH)加速生成,实现微波增强动态治疗(MEDT). Chang等[9]还构建了一种基于中空介孔Cu2MoS4负载葡萄糖氧化酶的多功能级联生物反应器,用于结合化学动态疗法、饥饿疗法、光热疗法、免疫疗法进行治疗癌症. Xu等[45]提出一种Cu2-xTe纳米酶用于肿瘤催化免疫疗法. 该纳米酶具有谷胱甘肽过氧化物酶活性,可以氧化GSH,改善肿瘤氧化应激,并且Cu2-xTe纳米酶还具有过氧化物酶活性,催化H2O2产生活性氧. 该纳米酶的缺陷结构导致其在近红外区有较强光吸收,从而可以实现很好的光热效应. Xu等[45]发现,在1 064 nm激光照射下,Cu2-xTe纳米酶不仅可以有效抑制原位瘤生长,还可以改变免疫抑制微环境,激活免疫治疗并建立免疫记忆来防止肿瘤复发. Huang等[46]设计了一种新型纳米酶,即硫铁矿(FeS2)纳米酶. 这种纳米酶结合H2O2底物的亲和力极高,催化H2O2的效率(Kcat/Km)比传统Fe3O4纳米酶高4 144倍,比天然辣根过氧化物酶高3 086倍. 该纳米酶不仅对H2O2有很高的亲和力,而且还同时具有谷胱甘肽氧化物酶的活性,可以氧化细胞中的还原型谷胱甘肽. 因此,FeS2纳米酶蕴含两种类酶活性,即谷胱甘肽氧化酶和过氧化物酶,构成级联反应,能够持续地产生羟基自由基,引起更多肿瘤细胞的凋亡.
此外,纳米酶在抗菌、细胞保护、治疗帕金森症等众多疾病治疗中都有应用. 虽然纳米酶在疾病治疗中显示出巨大的应用前景,但其生物安全性仍是一个问题. 纳米酶用于临床疾病治疗还有很长的路要走.
1.1. 纳米酶的制备方法
1.1.1. 贵金属纳米酶的制备方法
1.1.2. 金属氧化物纳米酶制备方法举例
1.1.3. 非金属纳米酶的制备方法
1.1.4. 有机金属框架纳米酶制备方法举例
1.2. 纳米酶的分类及其催化原理
1.2.1. 氧化酶
1.2.2. 辣根过氧化物酶
1.2.3. 过氧化氢酶
1.2.4. 谷胱甘肽过氧化物酶
1.2.5. 超氧化物歧化酶
1.2.6. 其他纳米酶
1.3. 纳米酶在生物医学方面的应用
1.3.1. 离子检测
1.3.2. 小分子检测
1.3.3. 核酸检测
1.3.4. 蛋白质检测
1.3.5. 疾病诊断
1.3.6. 疾病治疗
-
纳米酶在生物医学领域应用广泛,包括靶向癌症治疗、诊断医学、生物传感、环境毒理学等. 纳米酶在生物医学中的应用是一个比较新的研究领域,虽然发展迅速,但仍存在不少问题和挑战. 基于纳米酶在肿瘤靶向治疗方面的应用主要存在材料自身治疗效率不高和生物体或肿瘤微环境限制两个方面的问题. 一方面,一些纳米酶活性单一治疗效率低,而通过载体负载多种纳米材料的解决方案存在结构不稳定、制备过程复杂等问题. 因此,开发设计简单、多功能的“机器人”纳米酶以可控调节来提高治疗效率是非常必要的. 另一方面,肿瘤微环境的“土壤”也会在一定程度上限制其治疗效率. 因此,“因地制宜”根据肿瘤微环境的具体情况设计多功能纳米酶,用以调节肿瘤微环境同样重要. 另外,在一些纳米酶介导的化学动力疗法中,由于底物有限等制约因素限制了治疗效率的提高. 而纳米酶本身不仅可以模仿天然酶,其优异的物理化学性质还可以结合光、声等外部刺激实现多种治疗方式的协同作用,多样的酶活性也可为并行引入其他的治疗方式提供了丰富的可能性,一些纳米酶甚至还可以像在自噬机制中一样直接操纵特定的生物通路. 这些能力使纳米酶成为治疗和诊断应用的强有力工具. 但是如何精确设计,完美发挥其特定功能,“扬长避短”是关键.
无机纳米酶在医学领域还有很长的路要走,特别是临床应用,关键在于如何将其生物安全性提高到一个新的台阶. 在理想情况下,纳米颗粒在生物体内逐渐降解,最终排出体外,且不引起显著的生物毒性. 但是目前开发的用于癌症靶向治疗的纳米酶几乎都是生物体无法自然降解的,因为无机纳米粒子比有机可降解的纳米粒子具有更强的化学和机械稳定性. 对于纳米粒子的生物医学应用来说,这一特性是一把双刃剑,因为它虽然决定了纳米粒子的独特诊疗功能,但也引发了其生物安全性的问题. 因此,开发和利用可生物降解的纳米酶成为今后研究的主要方向之一.



 下载:
下载: