-
开放科学(资源服务)标志码(OSID):

-
光合作用是产量形成的基础,从能量转化角度讲,影响水稻产量潜力的主要因素包括:光吸收量、光能利用效率和收获指数[1],而当前水稻收获指数已接近理论上限[2],难以依靠优化收获指数来大幅提高水稻的产量. 因此,提高光合适应能力,促进生育前期获得较高光合物质基础,在维持较高收获指数基础上进一步提高光能利用效率以形成较高的生物量是实现水稻高产的重要途径[3-5].
西南地区是我国重要的水稻栽培区,但生育前期长期的阴雨寡日照、自然光照弱等客观因素致使冠层内叶片光合速率低,难以在生育前期获得较好的光合物质基础. 提高低光光适应能力进而提升低光光能利用效率成为该区域水稻光合适应性研究的重要目标. 水稻低光光能利用效率具有高度遗传特性,是影响水稻生物量形成的关键因素之一,且育成水稻品种之间低光光能利用效率存在很大变异,尚未在人工驯化过程中受到强烈选择[6]. 因此,低光光适应能力强的基因及资源材料挖掘成为进一步提高水稻产量的重要研究方向.
光合有效辐射(PPFD)不仅影响植物叶片的形态学特征变化(如光合色素质量分数)[7-8],还会对叶片的光合生理特征(如光合电子传递效率、光合碳同化效率)产生显著的调节作用[9-10]. 这些生理特征均会影响叶片的光合能力和光能利用效率. 除单叶水平光合效率外,保持合理的单株叶片面积也是提高作物群体光合和光能利用效率的重要基础,适宜的单株叶片面积可以显著改善群体光合物质的产出,进而促进作物产量的形成[11].
前期研究发现,嘌呤合成途径基因(Virescent-Albino Leaf 1,VAL1)编码蛋白VAL1,是嘌呤生物合成途径中的关键基因,参与调节水稻叶片发育过程中的叶绿体发育、叶绿素代谢和细胞分裂. 通过将VAL1在野生型材料中超表达,获得了光合速率显著提高且表型稳定的水稻株系(VAL1-OE)[12]. 本研究测定结果显示,VAL1-OE水稻具备同时适应低光和高光环境的典型生理特性,这表明VAL1-OE水稻同时拥有高效利用低光和高光的能力. 在自然界中,很少发现同时具备适应低光和高光能力的天然植物[13],这一特性使VAL1-OE水稻成为开展光合适应性研究的优势材料. 本文拟通过研究VAL1-OE与野生型材料在光合色素合成、光能吸收及光合碳同化效率等生理途径的差异,揭示VAL1-OE水稻增强光合适应能力及提高光合碳同化速率的调控机制,为挖掘寡日照地区水稻增产潜力,筛选光照广适稻种资源提供参考.
全文HTML
-
试验于2020年4月至8月在西南大学水稻研究所歇马(北纬N29°46′4.20″,东经E106°21′53.07″)试验基地进行. 试验选用缙恢10号野生型(WT)和VAL1超表达水稻(VAL1-OE)为试验材料,在抽穗期进行株型特征、叶片表型、光合作用及光能利用效率的测定与分析. 试验选取2个水稻材料,于4月育秧,5月6日移至田间插秧,栽插规格为株行距16.67 cm×26.66 cm,每个试验小区10行×10列,共100株,管理措施同大田. 试验区3 m高处运用防鸟网完全覆盖,以防止鸟类采食影响试验结果.
-
大田试验中,选择分蘖期水稻分别进行光合测定、色素质量分数测定和干物质分配测定.
水稻成熟后单独收种考种,水稻考种包括穗长、穗实粒数、穗空壳数等基本产量构成指标.
-
在抽穗期对水稻进行取样,每个试验小区取代表性植株3株,洗净根部泥土,然后测量相关指标. 测量指标包括株高、分孽数量、比叶质量(LMA)、单株叶面积(S)、叶片干质量(W)以及叶绿素质量分数,其中,单株叶面积(S)计算公式为
-
光合色素质量分数测定方法:用切割法切下部分叶片(用游标卡尺量叶片的长度并计算面积),然后加入10 mL叶绿素提取液,黑暗环境浸提48 h,每隔12 h混匀1次,最后用紫外分光光度测量吸光度. 测定时提前20 min将紫外分光光度预热,待预热结束后,以空白的叶绿素提取液作为对照,分别在645 nm,663 nm两个波长下测定提取液的吸光值(OD)[14].
OD645,OD663分别表示在波长645 nm,663 nm下测得的吸光值,Chla,Chlb,Chl(a+b)分别表示叶绿素a,叶绿素b和总叶绿素的浓度.
单位面积叶绿素质量分数(Cc,mg/m2)
式中,C为相应叶绿素的浓度(mg/mL),V为提取液体积(mL),S为测试样品的面积(m2).
-
用切割法切下部分叶片(用游标卡尺量叶片的长度并计算面积),先在烘箱中105 ℃杀青30 min,再在65 ℃烘至恒质量,最后用百分之一天平称质量.
-
水稻叶片A-PPFD的测定运用GFS-3000和双通道荧光仪(Chlorophyll Fluorescence & P700 Measuring System,DUAL-PAM-100,Walz,Germany)联用系统进行测定.
选取冠层上部倒1叶片为测试样本,样本叶片进行暗适应30 min后进行测定. 测定时,温度为27 ℃,相对湿度为70%,CO2浓度为400 μmol/mol,流速为400 μmol/s,光合有效辐射梯度设置为1 895,1 523,1 218,980,621,390,221,116,85,55,30,17,7和0 μmol/(m2·s). 选取1 523,980和390 μmol/(m2·s) 3个光强的点进行比较.
在测定A-PPFD时,叶绿素荧光和P700进行同步测定,光系统I和光系统II的电子传递速率ETR I和ETR II分别由试验仪器直接测定.
-
干物质的测定:将水稻各器官分别装于纸质信封,置于烘箱中105 ℃杀青30 min,然后在65 ℃烘干至恒质量后,用百分之一天平称质量.
产量及其构成因素的测定:水稻成熟前,调查每个小区的单株有效分蘖数,每个小区取10株进行考种,考种指标包括穗长、一次枝梗、二次枝梗、每穗实粒数和瘪粒数,计算结实率和单株产量.
-
采用Microsoft Excel 2010软件处理数据和作图,用SPSS 21.0软件进行统计分析. 同一试验参数不同处理间均进行差异显著性分析,p<0.05为差异有统计学意义. 文章中图、表数据均以x±s表示.
1.1. 试验概况
1.2. 样品采集及处理
1.3. 测定项目及方法
1.3.1. 株型指标测定
1.3.2. 叶绿素质量分数的测定
1.3.3. 比叶质量的测定
1.3.4. 光响应曲线(A-PPFD)的测定
1.3.5. 干物质、产量及其构成因素的测定
1.4. 数据分析
-
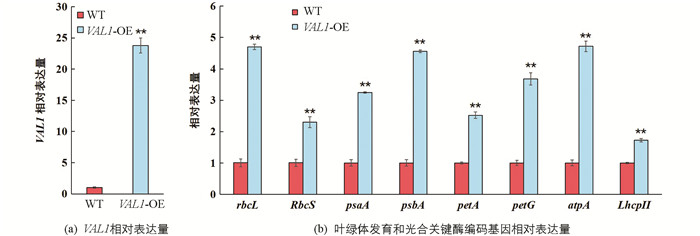
通过将VAL1基因在野生型材料缙恢10号(WT)中进行超表达获得了表型稳定的水稻株系VAL1-OE(图 1a),在分蘖期对野生型和VAL1-OE水稻叶片叶绿体发育和光合相关基因进行了qRT-PCR测定分析(图 1b). 试验结果表明,在光能吸收方面,VAL1-OE水稻叶片中编码捕光复合体II叶绿素a/b结合蛋白基因(LhcpII)的转录水平较野生型提高了75%;在电子传递方面,VAL1-OE水稻叶片中编码PS I P700叶绿素a脱辅基蛋白A1基因(psaA),PS II D1蛋白基因(psbA),细胞色素f脱辅基蛋白基因(petA)和细胞色素b6-f复合体小亚基基因(petG)的转录水平分别较野生型提高228%,355%,151%和258%;在光合羧化固定方面,VAL1-OE水稻叶片中编码核酮糖-1,5-二磷酸羧化酶/加氧酶大亚基基因(rbcL)、核酮糖-1,5-二磷酸羧化酶/加氧酶小亚基基因(RbcS)和叶绿体ATP合成酶α亚基基因(atpA)的转录水平分别较野生型提高了370%,131%和375%. 由此可知,VAL1-OE水稻叶片中调控光吸收、传递与转化过程主要基因的表达均显著上调(p<0.01).
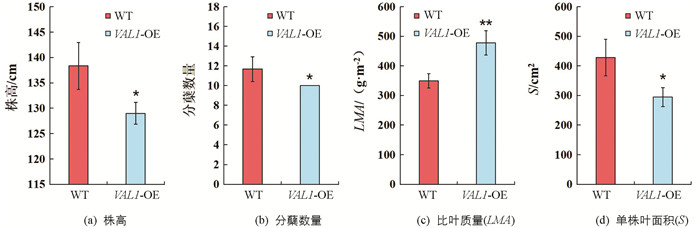
具有良好的株型特征是作物实现高光效的重要前提,分别对野生型(WT)和VAL1-OE水稻材料的株高、分蘖数量、比叶质量和单株叶面积等性状进行了差异显著性分析(图 2). 结果表明:VAL1-OE水稻材料的比叶质量(LMA)显著高于野生型,较野生型提高了36.95%(p<0.01,图 2c);VAL1-OE水稻材料的株高、分蘖数量和单株叶面积显著低于野生型(p<0.05),较野生型分别降低了6.75%(图 2a),14.29%(图 2b),和31.13%(图 2d).
-
VAL1超表达材料(VAL1-OE)与野生型(WT)之间的色素质量分数差异存在统计学意义(图 3). 试验结果表明,VAL1-OE水稻材料单位面积叶绿素a,叶绿素b和叶绿素(a+b)质量分数均显著高于野生型(p<0.05),分别较野生型提高了20.36%,8.09%和17.86%.
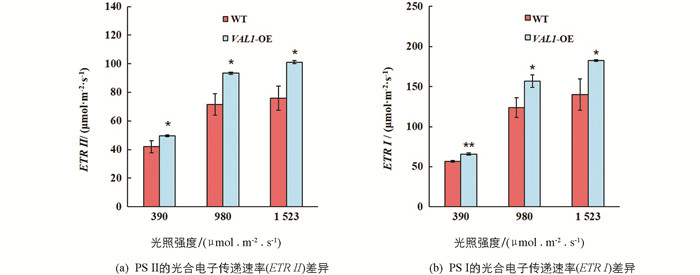
电子传递是光合作用进程中的重要环节,光合电子传递速率的不同也可能会对最终的光合值以及产量产生影响. 为探究VAL1-OE水稻材料与野生型(WT)之间光合电子传递的差异,分别在390,980和1 523 μmol/(m2·s)的光照强度下对野生型和VAL1-OE水稻材料的光系统II和光系统I电子传递效率进行差异显著性分析. 试验结果表明,在不同光照强度下,VAL1-OE水稻材料光系统II和光系统I电子传递效率均显著高于野生型(图 4). VAL1-OE水稻材料在390,980和1 523 μmol/(m2·s)的光照强度下光系统II电子传递效率分别较野生型提高了18.06%,30.76%和33.09%(p<0.05,图 4a);光系统I电子传递效率分别较野生型提高了16.14%,26.58%和30.28%(p<0.05,图 4b).
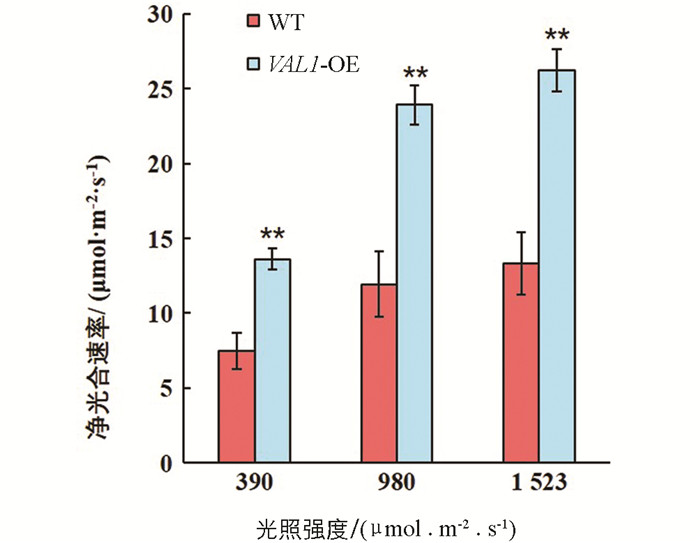
光合碳同化是植物干物质累积的基础,对植物生长发育具有重要的调控作用. VAL1-OE水稻材料与野生型(WT)在不同光强下的光合碳同化效率差异有统计学意义. 试验结果表明,在不同光照强度下,VAL1-OE水稻材料的净光合速率均显著高于野生型(p<0.01). 在390,980和1 523 μmol/(m2·s)的光照强度下分别提高了82.47%,100.43%和97.01%(图 5).
-
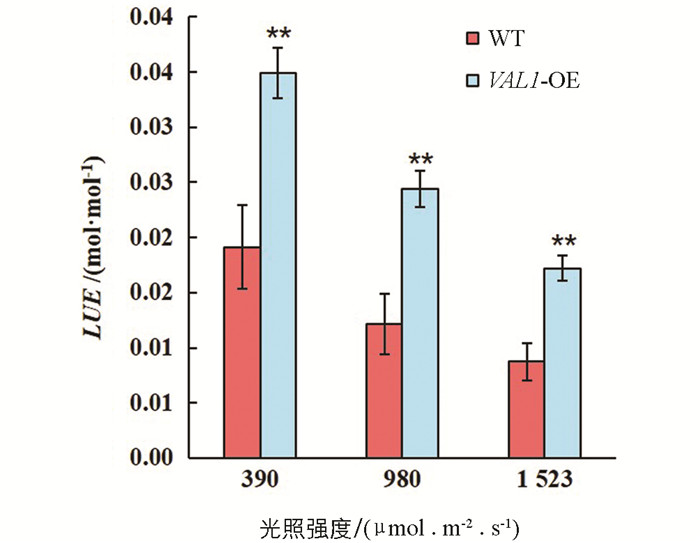
VAL1-OE水稻材料与野生型(WT)在不同光强下光能利用效率差异有统计学意义(图 6). 数据结果表明,在不同光照强度下,VAL1-OE水稻材料的光能利用效率均显著高于野生型(p<0.01),在390,980和1 523 μmol/(m2·s)的光照强度下的光能利用效率分别较野生型提高了82.47%,100.43%和97.01%. VAL1-OE水稻材料与野生型的考种数据结果表明,VAL1-OE水稻材料的抽穗期干物质量、穗长、实粒数、瘪粒数、穗数、千粒质量和总产量与野生型差异无统计学意义(表 1).
2.1. 基因表达和植型表型分析
2.2. 光合表型分析
2.3. 光能利用效率和物质累积及产量构成分析
-
光吸收能力的提高是优化光合作用的前提. 叶绿素a/b结合蛋白围绕光系统II(PS II)的反应中心作为主要的外部天线[15],能把接受的光能量快速传导至反应中心,参与光能转化与传递,以及对各种环境的适应等过程,对植物的光合作用起到关键作用[16]. 研究表明,在VAL1-OE超表达水稻叶片中,捕光复合体II叶绿素a/b结合蛋白基因(LhcpII),编码PS I P700叶绿素a脱辅基蛋白A1基因(psaA),PS II D1蛋白基因(psbA),细胞色素f脱辅基蛋白基因(petA)和细胞色素b6-f复合体小亚基基因(petG)的转录水平均显著提高(图 1),这些基因转录水平的提高有利于提升水稻叶片叶绿素a和叶绿素b的质量分数(图 3),使其在低光条件下能捕获更多的光能,促进光能的转化和传递(图 4),进而提高净光合速率和光能利用效率(图 5,图 6).
通常情况下,比叶质量较大的叶片也会具有较大的光合潜力. 研究结果表明,VAL1-OE超表达水稻叶片比叶质量显著高于野生型(图 2). 这有利于使叶片在单位面积上获得更多的氮素分配和光合蛋白的分布,进而提高VAL1-OE超表达水稻叶片在强光下的电子传递效率(图 4)、光合碳同化效率(图 5)和光能利用效率(图 6). 研究结果还表明,在强光条件下,VAL1-OE超表达水稻叶片ETR I显著高于ETR II(图 4),这主要是因为强光下水稻通过PS I的电子传递速率显著增强,进而导致叶片环式电子传递显著提高,而环式电子传递增强有利于高光强下实现对光合机构的保护作用[17]. 综上可知,VAL1-OE超表达水稻叶片关键光合酶编码基因转录水平的上调,显著提高了光合捕光复合蛋白的质量分数,促使无论是低光还是高光条件下,VAL1-OE超表达水稻叶片较野生型均具有显著的光合能力和光能利用效率.
-
具有良好的株型特征是作物实现高光效的基础. 张耀文等[18]在研究作物的高光效试验中认为,叶面积系数较大,株型紧凑且适于密植,茎秆粗壮抗倒伏能力强,冠层结构较良好,群体内光照分布合理、群体光合速率较高. 本研究结果表明,在抽穗期时VAL1-OE超表达水稻与野生型干物质累积量之间差异无统计学意义(表 1),但株高和分蘖数量显著低于野生型(图 2). 这可能是因为VAL1-OE超表达水稻叶片具有较强的光合能力,较多的干物质累积分配到各个分蘖当中,单分蘖获得了更多的干物质分配,致使其干物质总量未出现较大差异. 此外,在收获期时,VAL1-OE超表达水稻与野生型实际收获植株的分蘖数量差异无统计学意义,可能是由于进入抽穗期后,VAL1-OE超表达水稻重新获得一些新的有效分蘖增加了最终的有效穗数.
提高作物光能物质生产能力的途径有3个:提高光合能力、增大光合面积和延长光合作用的时间. 在本研究中,虽然VAL1-OE超表达水稻具有显著高的叶绿素质量分数、比叶质量和净光合速率(图 2,图 3,图 5),但VAL1-OE超表达水稻单株面积显著低于野生型(图 2). 光合面积较小成为制约VAL1-OE超表达水稻获得更多干物质累积和产量的主要因素. 以VAL1-OE超表达水稻为基础,在实现高光合能力的同时,培育高叶面积表型材料,提高光合作用面积是未来产量进一步提升的突破口.
3.1. 超表达VAL1水稻优化叶片光能吸收、电子传递和碳同化是提高光合作用和光能利用效率的关键
3.2. 在高光合基础上培育高叶面积表型材料,进一步提高光合作用面积是高光效水稻育种的突破口
-
超表达VAL1-OE水稻优化叶片光能吸收、电子传递和碳同化是提高光合作用和光能利用效率的关键. VAL1-OE超表达水稻叶片关键光合酶编码基因转录水平的上调,显著提高了光合捕光复合蛋白的质量分数,促使无论是低光还是高光条件下,VAL1-OE超表达水稻叶片较野生型均具有显著增强的电子传递速率和净光合速率,致使其光能利用效率显著提高. 与此同时,光合面积较小成为制约VAL1-OE超表达水稻获得更多干物质累积和产量的主要因素. 以VAL1-OE超表达水稻为基础,在实现高光合能力的同时,培育高叶面积表型材料,提高光合作用面积是进一步提高该水稻材料干物质累积量和产量的关键.



 下载:
下载: