-
香樟(Cinamomum camphora (L) Prest)为樟科、樟属亚热带常绿阔叶乔木[1].由于具有生长快、枝叶茂密、常绿且冠形美观,以及能避臭、驱虫、吸毒气、隔噪音等特点,香樟被广泛应用于城市园林绿化[2-3].随着香樟种植面积的扩大,香樟病害问题也越来越引起人们的重视.其中,香樟炭疽病是由盘长孢状刺盘孢(Colletotrichum gloeosporioides)引起的主要真菌病害[4],该病害在全国各地发病率极高,发病叶片呈现褐色病斑,且常伴随不规则形状的黑色小粒点[4-5].此外,炭疽病菌也能使香樟茎干枯死,影响其正常生长,给绿化建设带来极大的损失.目前,生产上防控香樟炭疽病以化学农药防治为主,另辅以合理修剪,树粮间作等农艺防控手段[6].然而,农艺法耗时费力,且防效甚微;化学法主要是通过多菌灵、红日强力杀菌剂、碘加酮杀菌剂等对香樟进行喷雾防治[7],尽管此法短期内防治效果较好,但易导致农药残留、病原菌产生耐药性、造成环境污染等一系列弊端.近年来,随着人们对环境问题的日益重视,使用安全有效的微生物菌剂[8-10]防治炭疽病已逐步成为一种极具潜力的生物防控手段,而利用此法进行香樟炭疽病防治的报道整体较少.因此,寻获能够有效抑制香樟炭疽病菌生长的微生物菌种资源对于绿色防控香樟炭疽病具有重要意义.
土壤微生物(soil microorganism)是生活在土壤中的细菌、真菌、放线菌及藻类等的总称[11].土壤有益微生物种类丰富,因其具有促进宿主植物生长[12]、增加宿主植物抗病虫害能力[13-14]等功能,被广泛应用于植物病虫害生物防治[15-17].近年来,有关利用土壤有益微生物防治植物炭疽菌的研究报道表明:土壤中存在大量能够抑制植物病原菌生长的拮抗菌群,这些菌群是开发植物病原微生物生防制剂的重要菌种来源[18-20].张晖等从香蕉根际土壤中分离获得几株对荔枝炭疽病菌与香蕉炭疽病菌均具明显抑菌作用的芽孢杆菌分离株[18];喇文军等从农田土壤分离筛选出的枯草芽孢杆菌C-D6对香蕉炭疽病原菌拮抗效果显著[19];祝福元等从土壤中分离获得的两株芽孢杆菌菌株19E2,13A1对菜心炭疽病菌具有强烈抑制作用[20].然而,有关利用土壤有益微生物防治香樟炭疽病的研究报道较少.
本研究对一株来自根际土壤的香樟炭疽病菌拮抗细菌进行菌种鉴定,优化该菌株产生抑菌活性物质的发酵条件,并测试其抑菌谱,以期为利用土壤有益微生物进行香樟炭疽病的防治奠定基础.
全文HTML
-
拮抗细菌SWUJ1菌株(来自根际土壤)及香樟炭疽病菌(C. gloeosporioides)SC菌株由重庆市风景园林科学研究院提供.
-
桑椹核地杖菌(Scleromitrula shiraiana) SXSG-5、核盘菌(Sclerotinia sclerotiorum) PZ-2、茎点霉(Phoma exigua) GXH1、锈腐病菌(Ilyonectria radicicola) SXZW10、鞭毛藻丛赤壳菌(Nectria haematococca) SXZW6为本实验室分离保存.腐霉菌(Pythium ultimum) SWU3、灰霉菌(Botrytis cinerea) SWU5、西瓜炭疽菌(Colletrichum lagenarium) SWU8、榆梢枯长喙霉(Ceratocystis ulmi) SWU10、立枯丝核菌(Rhizoctonia solani) SWU22、旋孢腔菌(Cochiobolus sativus) SWU25、链铬孢菌(Alternaria alternata) SWU26、尖孢镰刀菌(Fusarium oxysporum) SWU27为本实验室收集保存.
-
马铃薯葡萄糖琼脂培养基(PDA)、马铃薯葡萄糖培养基(PDB)、LB琼脂培养基:均参照文献[20]配制;使用PDB培养基作为氮源基础培养基;碳源基础培养基:马铃薯200.0 g煮沸20 min过滤取滤液,加入最佳氮源(根据氮源优化结果选择)10.0 g,蒸馏水定容至1 000 mL,pH值自然;无机盐基础培养基:马铃薯200.0 g煮沸20 min过滤取滤液,加入最佳氮源(酵母粉)10.0 g,最佳碳源(乳糖)20.0 g,蒸馏水定容至1 000 mL,pH值自然.将上述培养基在121 ℃的高压条件下灭菌20 min后备用.
-
基因组提取试剂盒(PrepMan Ultra Sample Preparation Regant)购自美国ABI公司;DNA marker、Taq酶、dNTP、PCR Buffer、Mg2+购自宝生物工程生工(大连)有限公司;PCR引物购自生工(上海)生物有限公司;生化鉴定试剂盒购自广东环凯生物科技有限公司;其他试剂均为国产分析纯.电泳仪、水浴锅均购自北京六一仪器厂,PCR仪购自美国ABI公司.
-
采用平板对峙法[21]检测抑菌活性:将目标拮抗菌新鲜培养物接种于PDB培养基,在28 ℃温度下,摇床转速为180 r/min,振荡培养24 h,获得菌悬液;活性检测时,用规格为5 mm的打孔器在长满新鲜炭疽病菌菌丝的PDA平板边缘处打取菌饼,接种至新的PDA平板中央,并在距菌饼中心30 mm对称处用菌悬液划一条直线,以不加拮抗菌菌悬液的PDA平板为对照,22 ℃恒温培养一周,观察抑菌带大小,以此判断拮抗细菌抑菌活性.
-
目标菌株培养特征、形态观察以及运动性、淀粉水解、明胶液化、硝酸盐还原等生理生化特性测试实验,均参照文献[22-24]的方法进行.
-
将SWUJ1菌株接种至LB液体培养基振荡培养12~18 h后,提取菌株基因组,并以其为模板,采用引物27F:5′-AGAGTTTGATCCTGGCTCAG-3′和1492R:5′-GGTTACCTTGTTACGACTT-3′进行16S rDNA扩增. PCR反应条件为:95 ℃ 5 min;94 ℃ 30 s,58 ℃ 30 s,72 ℃ 60 s,30个循环;72 ℃ 10 min.扩增产物送往生工(上海)生物有限公司测序,将获得的基因序列拼接后在NCBI(https://blast.ncbi.nlm.nih.gov/Blast.cgi)进行同源性比较分析,下载同源性较高的菌种序列信息,用ClustalX 2.0程序进行多重序列比对,利用MEGA 6.0软件邻接法(Neighbor-joining method)进行1 000次步长计算,构建系统发育树[25].
-
采用抑菌圈法[26]检测培养基成分(碳源、氮源和无机盐)及发酵条件(温度、pH值、接种量以及发酵时间)对SWUJ1菌株产生抑菌活性物质的影响.具体方法为:将SWUJ1菌株纯培养物接种至液体培养基恒温震荡培养后,收集发酵液,以10 000 r/min的转速离心30 min,取上清液过0.22 μm滤膜制备无菌发酵上清液.进而在新鲜PDA平板中央接种炭疽病菌SC菌株菌饼,并在距离菌饼中心30 mm处用8 mm直径的打孔器打孔,孔内加入200 μL拮抗细菌无菌发酵上清液,于25 ℃温度下培养1周,测量菌丝边缘至琼脂孔的距离,以此数值表示抑菌带宽度,判断拮抗细菌相应发酵条件下的抑菌活性大小.
-
(1) 氮源优化:在其他条件不变的情况下,在氮源基础培养基(PDB培养基)中分别加入1%的蛋白胨、酵母粉、牛肉膏、胰蛋白胨、(NH4)2SO4、NH4NO3、NaNO3作为氮源,以氮源基础培养基为对照,取目标菌株种子液加入发酵培养基中(接种量1%),于28 ℃、摇床转速为180 r/min的条件下振荡培养96 h,每个处理设置3次重复.
(2) 碳源优化:在其他条件不变的情况下,在碳源基础培养基中分别加入2%的葡萄糖、蔗糖、麦芽糖、乳糖、可溶性淀粉和苹果酸作为碳源,以碳源基础培养基为对照,取目标菌株种子液加入发酵培养基中(接种量1%),于28 ℃、转速为摇床转速为180 r/min的条件下振荡培养96 h,每个处理设置3次重复.
(3) 无机盐优化:在其他条件不变的情况下,在无机盐基础培养基中分别加入0.05%,0.10%,0.15%共3个质量分数梯度的KCl,MnSO4,KH2PO4,Na2HPO4,NaCl,FeSO4和MgSO4,以无机盐基础培养基为对照,取目标菌株种子液加入发酵培养基中(接种量1%),于28 ℃、摇床转速为180 r/min的条件下振荡培养96 h,每个处理设置3次重复.
-
基于已经优化的发酵培养基组分,检测SWUJ1菌株在不同培养条件下获得发酵液的抑菌效果,每种条件设置3个重复.
(1) 温度:设置20,25,28,30,32,35,37和40 ℃共8个温度,其他条件一致,接种后于180 r/min条件下培养3 d,检测抑菌效果,确定最适温度;
(2) 起始pH值:设置3.0,4.0,4.5,5.0,6.0,7.0,8.0和9.0共8个起始pH值,其他条件一致,接种后于28 ℃、摇床转速为180 r/min的条件下培养3 d,检测抑菌效果,确定最适起始pH值;
(3) 发酵时间:设置12,24,36,48,72,96,120,144 h共8个发酵时间,其他培养条件一致,于28 ℃、摇床转速为180 r/min的条件下培养后,检测抑菌效果,确定最适发酵时间;
(4) 接种量:设置1%,2%,3%,4%,5%,6%,7%,8%和9%共9个接种量,其他培养条件一致,于28 ℃、摇床转速为180 r/min的条件下培养3 d,检测抑菌效果,确定最适接种量.
-
将拮抗菌株SWUJ1接种于优化后的发酵培养基,按1.2.3中的方法制备无菌上清发酵液,用菌丝生长速率法[27]检测其对核盘菌PZ-2、旋孢腔菌SWU25等10余种植物病原菌的抑菌效果.根据病原菌生长速度,腐霉病菌SWU3及尖孢镰刀菌SWU27在培养1 d时测量抑菌率,桑椹核地仗菌SXSG-5、锈腐病菌SXZW10、茎点霉GXH1及西瓜炭疽菌SWU8在培养8 d时测量抑菌率,其余病原真菌在培养3 d时测量抑菌率.具体方法为:将PDA培养基高压灭菌后冷却至50 ℃左右,以1%的比例混入拮抗菌株SWUJ1无菌发酵上清液制备检测平板,以未加发酵滤液的PDA平板作为对照,接入直径为5 mm的病原菌菌饼后,置于25 ℃培养箱中培养,每个处理组3个重复.实验结束后,用十字交叉法测量病原菌菌落直径,并计算抑菌率[28]:
-
实验数据用Office excel 2010处理,使用SPASS 17.0软件进行单因素方差统计分析,结果以“平均值±标准误差”表示.
1.1. 材料
1.1.1. 供试菌株
1.1.2. 供试抗菌谱病原菌
1.1.3. 培养基
1.1.4. 主要试剂与仪器
1.2. 方法
1.2.1. 抑菌活性检测方法
1.2.2. 拮抗细菌鉴定的方法
1.2.2.1. 菌体培养特征、形态观察及生理生化特征测定
1.2.2.2. 菌体分子生物学鉴定和系统发育分析
1.2.3. 拮抗菌株发酵条件优化
1.2.3.1. 培养基成分优化
1.2.3.2. 培养条件优化
1.2.4. 拮抗菌株抑菌谱的检测方法
1.3. 数据分析
-
抑菌测试结果表明,SWUJ1菌体对炭疽病菌SC菌株具有较强的抑制活性.香樟炭疽病菌SC菌株在对照PDA培养基上生长迅速,1周后长满整个平板(图 1(a)),而炭疽病菌SC菌株与SWUJ1菌株在PDA培养基上对峙培养时,出现明显的抑菌带(图 1(b)).
-
将拮抗菌SWUJ1菌株接种至LB固体培养基,28 ℃恒温培养24 h,观察到菌落呈圆形、边缘整齐光滑、湿润、呈乳白色黏稠状(图 2(a)). SWUJ1菌株革兰氏染色与芽孢染色结果表明,该菌体为革兰氏阳性杆状菌株,且能产芽孢(图 2(b)、图 2(c)).
-
生理生化实验结果表明,SWUJ1菌株能够还原硝酸盐、液化明胶、水解淀粉、过氧化氢酶检测呈阳性;能够利用枸橼盐酸,在半固体琼脂培养基中能扩散生长,表明其具有运动性(表 1).这些特性与芽孢杆菌属菌株的生理生化特性一致,综合对SWUJ1菌株的形态学观察及生理特性分析,判断其为芽孢杆菌属菌株.
-
将SWUJ1菌株16S rDNA扩增产物送至生工(上海)有限公司测序,得到长度为1 454 bp的片段,提交该序列相关信息至GenBank,获得登录号MH613286,将该序列与GenBank中序列进行在线比对,结果显示其与多株Bacillus methylotrophicus菌株16S rDNA序列的同源性高达99%.基于16S rDNA系统发育分析结果显示,SWUJ1菌株登录号为NR116240的甲基营养型芽孢杆菌的亲缘关系最近,处于系统发育树的同一分枝(图 3).
结合SWUJ1菌株的形态学观察、生理生化反应特征、16S rDNA序列的系统发育分析结果,鉴定SWUJ1菌株为甲基营养型芽孢杆菌(Bacillus methylotrophicus),命名为B. methylotrophicus SWUJ1.
-
通过单因素试验检测不同碳源、氮源以及无机盐对拮抗菌株产抑菌活性物质的影响.结果表明,在选用的7种氮源培养基上生长的SWUJ1菌株均可产生抑菌活性物质.其中以酵母粉为氮源时,该菌发酵液的抑菌活性最大,抑菌带宽度为11.97 mm,比对照组提高了60.99%,其次是(NH4)2SO4、NH4NO3、牛肉膏、蛋白胨、NaNO3、胰蛋白胨(图 4(a)),故酵母粉为最佳氮源;在选用的6种碳源培养基上生长的SWUJ1菌株,除苹果酸外,其余均可产生抑菌活性物质.其中以乳糖为碳源时,该菌发酵液的抑菌活性最大,抑菌带宽度为12.26 mm,比对照组提高了45.45%,其次是可溶性淀粉、葡萄糖、蔗糖、麦芽糖(图 4(b)),故乳糖为最佳碳源;在选用的7种无机盐中,采用质量分数为0.10%的MgSO4最有利于SWUJ1菌株产生抑菌活性物质,抑菌带宽度可高达15.17 mm(图 4(c)).
综上所述,优化后的培养基配方为:马铃薯200.0 g煮沸20 min过滤取滤液,加入酵母粉10.0 g,乳糖20.0 g,MgSO4 1.0 g,蒸馏水定容至1 000 mL.
-
在确定培养基成分优化结果的基础上,对SWUJ1菌株发酵条件进行单因素优化试验.结果表明:与其他培养温度相比,SWUJ1菌株在25 ℃培养条件下,发酵液抑菌活性最大,其抑菌带宽度可达16.43 mm(图 5(a));pH值过高或过低,均会显著降低发酵液的抑菌活性,初始pH值为5.0时抑菌活性最强(图 5(b)),其抑菌带宽度可达16.93 mm;而种子液接种量对菌株发酵液抑菌活性影响不大,种子液接种量为1%时,发酵上清液抑菌活性略强(图 5(c));同时,不同发酵时间对菌株产抑菌活性物质的影响显著,随着培养时间延长,发酵液抑菌活性逐渐增加,待到菌株培养至96 h时,其抑菌活性最强,抑菌带宽度可达16.73 mm(图 5(d)),随后,培养时间过长抑菌活性呈现下降趋势.综上可知,拮抗菌株SWUJ1的最适培养温度为25 ℃,最适初始pH值为5.0,最适初始接种量为1%,最适培养时间为96 h.
利用优化后的培养基配方和发酵条件培养SWUJ1菌株,进行优化结果的验证.结果表明,优化后SWUJ1菌株等量发酵上清液的抑菌带宽度((16.64±0.09) mm)显著大于PDB培养基发酵上清液的抑菌带宽度((7.435±0.09) mm),抑菌效果提高了123.81%,优化后的发酵培养基组成与发酵条件有利于抑菌活性物质的生成(图 6).
-
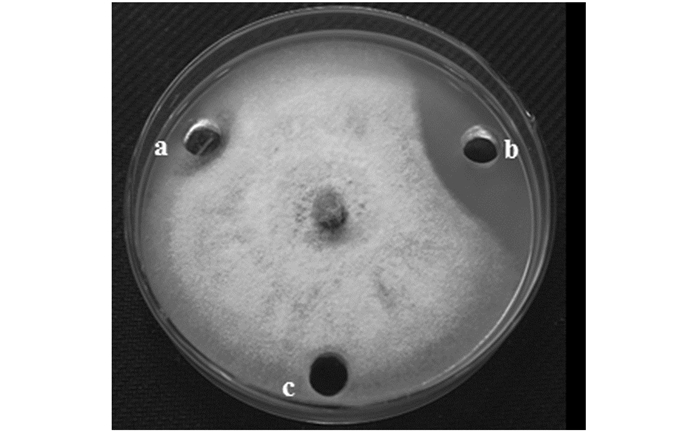
抑菌谱测试结果表明,SWUJ1菌株对多种植物病原菌具有较强的抑制作用,尤其对桑椹核地仗菌SXSG-5、核盘菌PZ-2和旋孢腔菌SWU25的抑菌效果最为显著,抑菌率分别高达100%,100%和94.89%(表 2).因此,SWUJ1菌株有望开发成为植物真菌病害的生防菌株.
2.1. 拮抗菌菌体的抑菌活性检测结果
2.2. 拮抗菌株SWUJ1的鉴定
2.2.1. 形态学鉴定
2.2.2. 生理生化实验
2.2.3. SWUJ1菌株16S rDNA序列测定及系统发育分析
2.3. SWUJ1菌株的发酵条件优化
2.3.1. 培养基成分的优化
2.3.2. 培养条件的优化
2.4. 拮抗菌株SWUJ1抑菌谱检测
-
本研究对一株来自根际土壤的香樟炭疽病菌拮抗细菌SWUJ1菌株进行菌种鉴定,并对其抑菌活性物质进行发酵条件的优化,结果表明其优化后的无菌发酵液对香樟炭疽病病原菌的抑菌率提高了123.81%.根据该菌株形态特征、生理生化检测结果及基于16S rDNA的系统发育分析将其鉴定为甲基营养型芽孢杆菌(Bacillus methylotrophicus),且SWUJ1菌株对核地仗菌SXSG-5、核盘菌PZ-2及旋孢腔菌SWU25等植物病原菌亦表现出较强的抑菌活性,表明该菌株具有较宽的抑菌谱.
芽孢杆菌是一类好氧或兼性厌氧、能形成抗逆性内生芽孢的杆状细菌,广泛分布于土壤、水体及植物体等多种生态环境中[29-32].大多数芽孢杆菌可通过生成抑菌活性物质[33],竞争性排阻[34],诱导宿主植物产生抗病性及忍耐性[35]等多种机理抑制植物病原菌的生长,从而提高宿主植物的抗病性能.甲基营养型芽孢杆菌(Bacillus methylotrophicus)作为芽孢杆菌属的重要种群,广泛分布于土壤环境中,并发挥着潜在的生防作用.常征等从三七园土壤中分离的甲基营养型芽孢杆菌YS(r)-1对三七腐霉菌具有较强的抑制作用[36];孙卓等从森林土壤中筛选获得的甲基营养型芽孢杆菌SZ-2可用于人参锈腐病的防治[37];尹向田等从葡萄种植园中分离出的甲基营养型芽孢杆菌GSBM05对葡萄白腐病菌等10余种植物病原菌有抑菌效果[38].本研究中的甲基营养型芽孢杆菌SWUJ1菌株亦展现出对香樟炭疽病菌的生物防治潜能.
抑菌活性物质的生成是生防菌株发挥防病功能的重要机制之一,而活性物质的生成效率不仅与菌株自身遗传特性相关,亦受菌株培养条件影响.为提高SWUJ1菌株抑菌活性物质的产量,本研究对其发酵条件进行优化,优化后的培养基成分和培养条件能够显著提高SWUJ1菌株等量发酵液的抑菌活性,为香樟炭疽病防治提供了新的菌种资源.后续研究将进一步分离纯化B. methylotrophicus SWUJ1菌株发酵液中的主要抑菌活性物质,探究其对香樟炭疽菌具有抑制作用的相关机制及其在室外的防治效果,为在生产中利用该菌株进行香樟炭疽病的生物防治奠定坚实的研究基础.




 下载:
下载: