-
开放科学(资源服务)标志码(OSID):

-
奥氮平(Olanzapine,Ola)是二代抗精神病药物的典型代表,但长期使用容易导致患者体质量增加、肥胖及其他代谢性疾病,如高脂血症、2型糖尿病等;Ola诱发的脂代谢紊乱因控制不理想、且缺乏相应的预防措施致使心脑血管并发症和早逝风险大为增加[1-4]. 现阶段,Ola介导代谢副反应的具体机制尚未明确,各研究团队以啮齿动物为模型,选择0.5~6 mg/kg等剂量,探究了这类药物介导代谢副反应的相关机制[5-9]. 但因实验设计中,给药方式、剂量、作用时间等差异大,研究结论仍存在诸多不一致.
本研究旨在模拟临床口服给药方式,探究临床用药剂量范围内,药物剂量与代谢副反应之间的关联,并进一步解析Ola介导脂代谢紊乱的可能机制,以期为合理建立动物实验模型及解决临床问题提供一定的指导.
HTML
-
SPF级健康雌性成年SD大鼠50只,8周龄,体质量180~220 g,来自重庆市中药研究院实验动物研究所,实验动物生产许可证:SCXK(渝)2007-0006. 大鼠每笼2只,于室温22 ℃,相对湿度(50±5)%且模拟昼夜规律(7:00~19:00开启照明)的环境下饲养;适应环境3 d后,训练主动口服糖丸3 d(主动口服给药). 所有动物实验经学院实验动物伦理委员会批准实施.
-
Ola,美国Lilly药业公司,C17H20N4S,CAS号:132539-06-1. 按比例将玉米淀粉(47.3%)、蔗糖(30.9%)、明胶(6.3%)和干酪素(15.5%)混合均匀后制成空白糖丸;将剥去包衣的Ola片剂用研钵磨成粉,按不同药物浓度称取相应质量的粉末加入到空白糖丸干粉混合物中制成含药糖丸,4 ℃避光储存. 以2.0 mg/kg剂量组(总质量2.5 kg)每天给药糖丸制作为例:取1片奥氮平片(有效量为5mg)去除包衣研磨成细粉后,加入按比例混合均匀的空白糖丸干粉15 g,再次充分混匀后加水制成软糯的糖丸,并按照每只大鼠对应体质量占比称取相应质量的糖丸,将其均分为3份.
-
油红O,北京鼎国昌盛生物公司(DH222-1);总RNA快速提取试剂盒,北京百泰克生物技术有限公司(RP1202);ChamQ SYBR qPCR Master Mix,南京诺唯赞(Q321-02);HiScript III RT SuperMix for qPCR,南京诺唯赞(R323-01);所有血清生化指标检测试剂均购自北京鼎国昌盛生物公司;抗体SREBP1,Bioss bs-1402R;OCTN2,ABclonal (A1676);CPT1A,ABclonal (A5307);PPARα,Wanleibio (WL00978);GAPDH(胞浆内参蛋白),Servicebio (GB11002).
-
生化分析仪,Olympus AU400 chemistry analyser,Japan;冰冻切片机,德国莱卡公司(CM1900);酶标仪,Bio-Rad (Model 680);实时荧光定量PCR仪,Bio-Rad;PCR仪,Bio-Rad;荧光及化学发光成像系统,上海勤翔(ChemiScope 6000 Pro).
1.1. 实验动物
1.2. 药品
1.3. 主要试剂
1.4. 主要仪器
-
将大鼠随机分为5组,适应环境及口服糖丸训练结束后,分别给0,0.25,0.5,1.0,2.0 mg/kg的含药糖丸,每天3次,每次间隔8 h,连续给药14 d. 饲养及给药期间,每天记录大鼠的体质量、摄食和饮水情况. 药物处理第14 d,所有动物禁食12 h后(过夜),经二氧化碳窒息处死,心脏采血,并分别测量大鼠的肾周脂肪、卵巢周围脂肪、腹股沟脂肪、总腹部脂肪和总白色脂肪的质量[10].
-
心脏采血,将含有促凝剂的全血放入37 ℃水浴30 min,1 200 r/min离心10 min,留上清并保存至-20 ℃冰箱;检测血清总胆固醇(TC)、甘油三酯(TG)等参数;检测10%肝脏匀浆中游离脂肪酸(FFA)量.
-
利用冰冻切片机获取12 μm厚度的肝脏组织切片,复温15 min,用油红O染色10 min,流水冲洗去除多余的油红O染液,最后用苏木素染核10 s;腹部白色脂肪经石蜡包埋切片后获得4 μm的组织切片,采用常规HE染色方法进行染色;染色结果于显微镜下观察并拍照,相应指标用Image Pro Plus(IPP)进行统计.
-
采用H-DMEM(含10%胎牛血清)培养HepG2细胞至密度为80%~90%,铺板过夜后由Ola(0,2.5,5,10 μmol/L)处理24 h. 磷酸缓冲液(PBS)清洗,用4%多聚甲醛固定细胞15 min;用含0.5%Triton X-100的PBS室温通透细胞20 min,再用1% BSA封闭1 h;经4 ℃一抗(1∶50)孵育过夜,37 ℃避光孵育二抗(CY3)1 h;DAPI(4′,6-二脒基-2-苯基吲哚) 孵育5 min,荧光显微镜下观察拍照,合并后得到MERGE图像.
-
按试剂盒方法提取肝脏总RNA,取1 μg RNA逆转录得到cDNA,用SYBR进行荧光定量. 每个样本做3个平行,以Gapdh和Rpl13a为内参基因,以2-ΔΔCt法计算mRNA相对表达量.
-
肝脏组织加入蛋白裂解液后(含PMSF)在均质破碎仪上匀浆,冰上裂解30 min,离心取上清,用BCA法测蛋白浓度. 蛋白样品经SDS-PAGE电泳分离,转印到PVDF膜上,5%脱脂奶粉封闭,4 ℃孵育一抗过夜,室温孵育二抗1 h,ECL化学发光显影,Quantity One统计灰度值[11],每个样本重复2次实验,GAPDH为胞浆内参蛋白,H3为核内参蛋白.
-
采用SPSS 19.0统计软件进行分析,Kolmogorov-Smirnov test检测所有数据分布;组间比较采用单因素方差分析,所有数据用x±s表示,p<0.05表示差异有统计学意义.
2.1. 药物处理
2.2. 生化分析
2.3. 组织油红O(ORO)染色及苏木精伊红(HE)染色
2.4. 细胞免疫荧光
2.5. 总RNA提取及RT-PCR检测
2.6. 蛋白提取及Western blot检测
2.7. 数据处理和统计分析
-
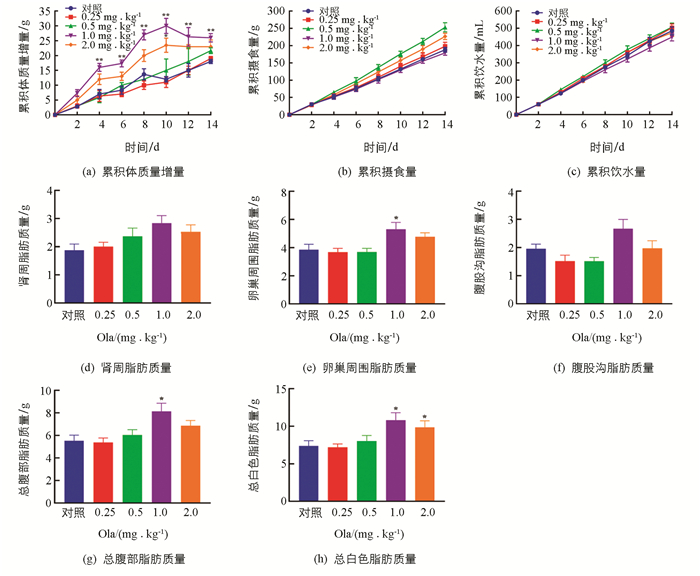
Ola处理2周后,给药组体质量累积增量均高于对照组,其中1.0和2.0 mg/kg剂量组体质量增加明显(p<0.01和p<0.05);摄食、饮水未见明显改变. 此外,对各剂量组的脂肪组织质量进行评估发现:1.0 mg/kg剂量组大鼠总腹部脂肪、卵巢周围脂肪以及总白色脂肪显著增加(p<0.05);2.0 mg/kg剂量组大鼠总白色脂肪显著增加(p<0.05)(图 1).
-
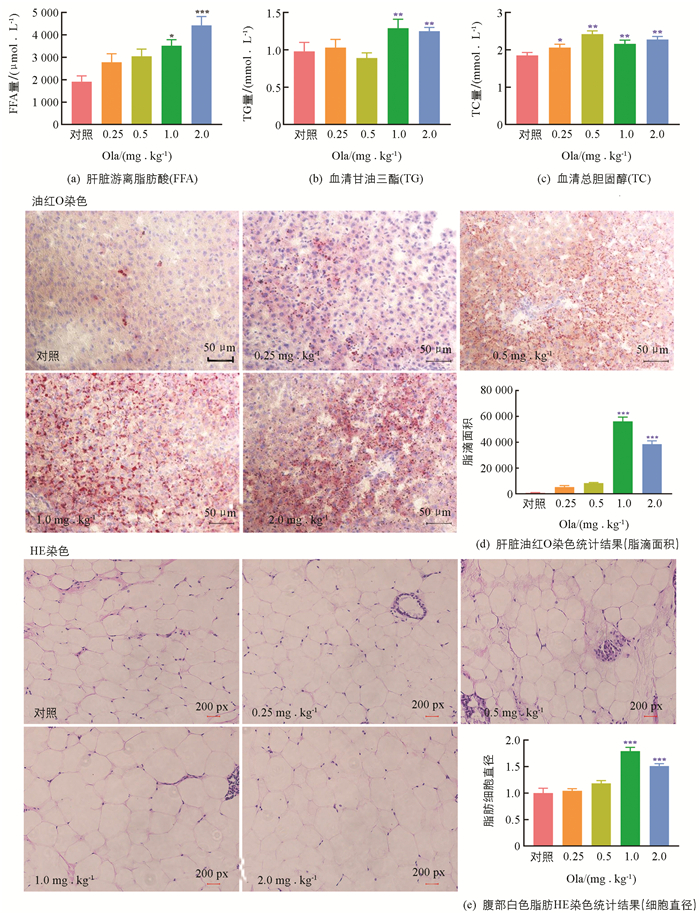
药物处理2周后,大鼠肝脏游离脂肪酸(FFA)量随给药剂量递增呈上升趋势,其中1.0和2.0 mg/kg剂量组FFA增加与对照组相比差异有统计学意义;1.0和2.0 mg/kg剂量组大鼠血清TG水平增加与对照组相比差异有统计学意义(p<0.01);各剂量组TC水平与对照组相比,差异均有统计学意义. 对肝脏进行切片油红O染色,给药2周后,1.0和2.0 mg/kg剂量组脂滴面积显著高于对照组. 腹部白色脂肪切片HE染色结果显示,1.0和2.0 mg/kg剂量组脂肪细胞直径显著高于对照组(图 2).
-
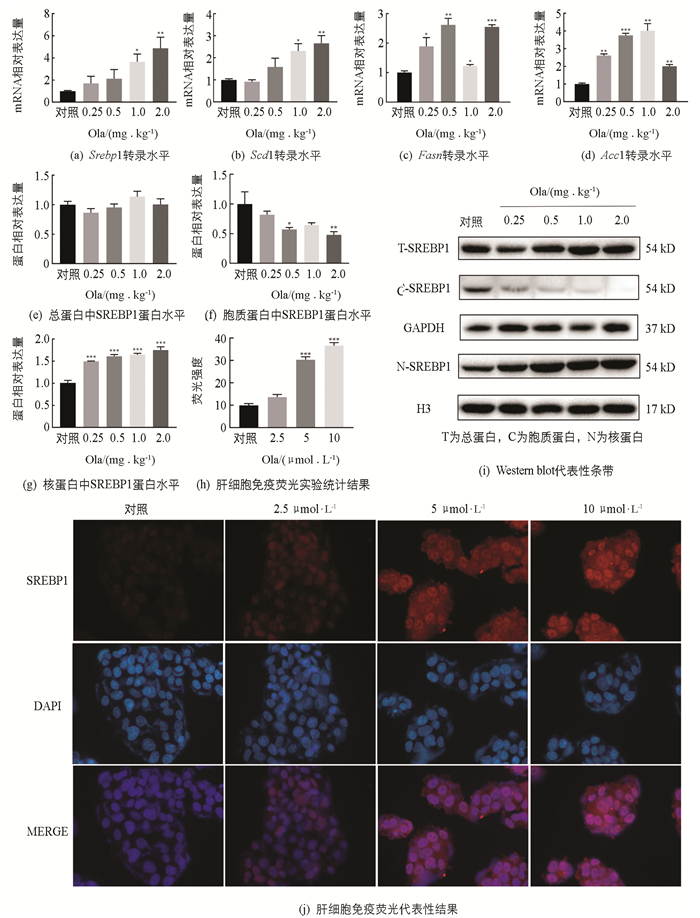
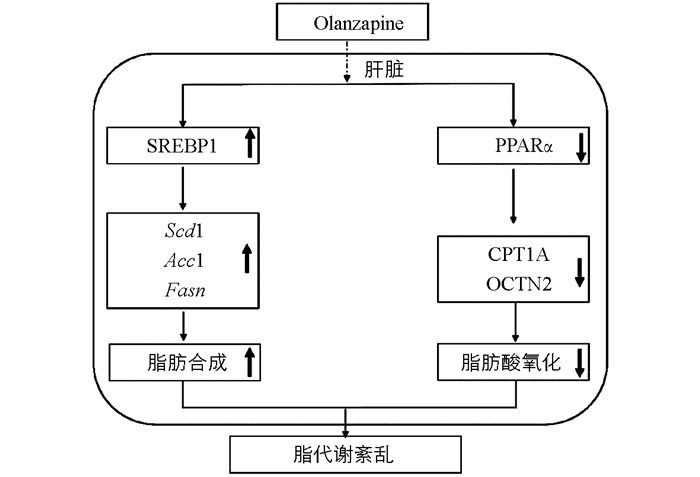
脂质的堆积可能是由于脂肪合成增多,肝脏是脂代谢的重要场所,因此需要对肝脏中脂肪合成相关因子进行探究(图 3). 实时荧光定量PCR实验结果表明:在给药2周后,与对照组相比,脂肪合成相关因子Srebp1,Scd1,Fasn和Acc1 mRNA水平显著上调,不同给药剂量上调程度有所不同;总蛋白中SREBP1蛋白表达水平与对照相比变化无统计学意义,药物干预后胞质蛋白中SREBP1蛋白表达水平下降,核蛋白中的SREBP1显著增加(p<0.001),肝细胞免疫荧光结果也显示细胞核中的SREBP1表达水平随Ola剂量增加而增加.
-
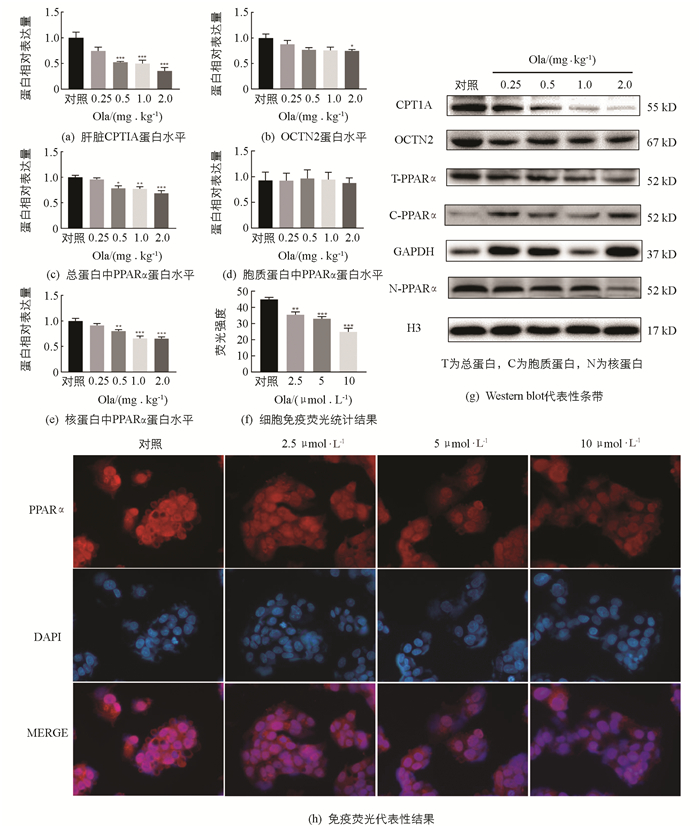
检测Ola对肝脏中脂肪酸氧化相关因子的影响(图 4),结果显示:Ola给药2周后,0.5,1.0和2.0 mg/kg剂量组肝脏中脂肪酸氧化相关因子CPT1A和总蛋白中PPARα显著下调,同时核蛋白中PPARα也显著下调,且随Ola剂量增加下调程度增大,胞质蛋白中PPARα变化无统计学意义;细胞免疫荧光实验结果显示细胞核中PPARα剂量下调;OCTN2也出现了下调的结果,其中2.0 mg/kg剂量组变化有统计学意义(p<0.05).
3.1. 奥氮平给药影响大鼠体质量及脂肪生成
3.2. 奥氮平给药影响大鼠脂质代谢
3.3. 奥氮平上调大鼠脂肪酸合成相关因子
3.4. 奥氮平下调大鼠脂肪酸氧化相关因子
-
根据《精神分裂症防治指南》(2版),本实验模拟临床上急性期患者诊疗方案,设计了0.25,0.5,1.0和2.0 mg/kg奥氮平4个剂量组(等同于临床每60 kg用药5~20 mg)每14 d的药物处理实验. 实验结果显示,在临床剂量范围内,Ola剂量与脂代谢副反应程度之间相关联,不同剂量Ola会介导不同程度的代谢副反应. 1.0 mg/kg和2.0 mg/kg(等同于临床常用的每60 kg用药10 mg和20 mg)剂量组的各项检测指标最为显著. 因此,在这类药物的使用过程中,临床监测是非常必要的.
实验过程中,为了尽量减少大鼠因灌胃、注射等给药方式而产生的应激反应,我们采用了主动口服给药,使之更接近临床病人用药的方式[12-13]. 此外,在药物作用时间上,因临床研究发现,抗精神病药导致体质量增加及糖脂代谢变化不同于单纯性肥胖,其糖脂代谢有其自身的特点,尤其在治疗干预前2~4周,体型指标及血脂指标出现显著变化[14-15]. 因此,本研究选择了14 d的给药时间,等同于临床4~6周治疗时间. 结果发现,药物处理14 d后,动物出现显著的体质量增加和脂代谢变化,尤其是1.0 mg/kg和2.0 mg/kg剂量组. 因此,我们认为SD大鼠能较好地模拟药源性代谢紊乱,这为进一步分子机制的探析提供了稳定的动物模型.
SREBP1是调节肝脏脂代谢的关键因子[16-17],与脂肪合成密切相关[18-20],本实验结果表明,Ola通过增加肝脏中SREBP1入核,激活下游脂肪合成相关靶基因Fasn,Acc1和Scd1的转录,增加脂质合成. PPARα,CPT1A,OCTN2与肝脏脂肪酸氧化相关[20-24],CPT1A是脂肪酸β氧化的限速酶;OCTN2作为最主要的肉碱转运蛋白[21],负责肉碱的摄取转运,也是脂肪酸β氧化的关键因子,已经发现补充肉碱可以在一定程度上减轻抗精神分裂症药介导的肝脏脂质积聚[22],进一步说明OCTN2在抗精神分裂症药介导的脂代谢紊乱中的关键作用;PPARα是脂肪分解过程中的关键转录调控因子,参与CPT1A和OCTN2的转录调控[25-26]. 我们发现奥氮平通过减少PPARα的入核,下调下游脂肪酸氧化关键因子CPT1A和OCTN2的蛋白表达,抑制脂肪酸的β氧化,脂质分解减弱. Ola介导的脂质代谢紊乱是脂质合成增多及分解减小共同作用的结果.
-
实验结果显示:2周Ola给药处理后,SD大鼠出现脂质代谢紊乱,该效应与Ola剂量存在显著相关,尤其是临床常用剂量(1.0 mg/kg和2.0 mg/kg)存在较大的诱发脂代谢紊乱的风险. 该副反应可能与Ola上调脂肪合成因子SREBP1及其下游靶标基因Fasn,Acc1,Scd1和下调脂肪酸氧化相关因子PPARα,CPT1A和OCTN2有关(图 5).






 DownLoad:
DownLoad: