-
开放科学(资源服务)标志码(OSID):

-
核盘菌(Sclerotinia sclerotiorum)属子囊菌门(Ascomycota)、核盘菌科(Sclerotiniaceae)、核盘菌属(Sclerotinia)真菌,能够广泛侵染包括甘蓝型油菜(Brassica napus)在内的400多种植物[1]. 甘蓝型油菜是重要油料作物之一,广泛种植于中国、加拿大及欧洲等温带地区[2-3],供给全世界约13%的植物油,是全世界传统食用油和能源用油的重要来源. 核盘菌侵染所致的菌核病可引起油菜减产10%~20%,严重时可达80%[4]. 目前,油菜菌核病的防控方法各有局限,如化学防治不仅导致农药残留、病菌的抗药性增强、生态环境恶化,还可引起农业生产成本增加[5-6]. 菌核在土壤中存活时间长,且其寄主植物范围广,作物轮作也很难对其实现有效控制[7]. 栽培油菜中缺乏抗病资源,也制约其抗性育种进程[8]. 近年来,生物防治逐渐成为菌核病防控的新策略,核盘菌的生防制剂研究也取得了一些进展,比如盾壳霉(Coniothyrium minitans)能抑制核盘菌子囊孢子对油菜叶片的侵染,并减少其子实体的产生数量,有效降低油菜菌核病的发病率[9-11]. 印度梨形孢(Piriformospora indica)由Kost等[12]发现于印度沙漠地区灌木丛根部,因其孢子类似梨形而得名. 印度梨形孢的共生不仅有利于植物对营养元素的吸收[13-15],促进植物生长[16-18],还对部分病原真菌有抑制作用. 印度梨形孢能够抑制大麦布氏白粉病菌(Blumeria graminis f. sp. tritici)[19]、小麦基腐病菌(Pseudocercosporella herpotrichoides)[20]、黄萎病菌(Verticillium dahliae)[21]、串珠镰孢菌(Fusarium verticillioides)[22]以及灰霉病菌(Botrytis cinerea)[23]等,可能是一种重要的植物病害生物防控资源. 灰霉病菌与核盘菌属真菌近缘,尤其与核盘菌亲缘关系较近[24]. 基于印度梨形孢可以有效抑制灰霉菌,我们推测其可能也是核盘菌生物防治的潜在资源. 为此,本研究对核盘菌与印度梨形孢进行对峙培养,探究印度梨形孢对核盘菌的体外抑制作用,并分析对峙培养过程中核盘菌生长发育、逆境胁迫响应关键基因的表达水平,解析其抑制作用机理,为利用印度梨形孢对菌核病进行生物防治提供基础.
HTML
-
试验于2019-2020年在重庆市油菜工程技术研究中心进行.
-
核盘菌“1980”由重庆市油菜工程技术研究中心保存,参考Mei等[25]的方法进行活化与继代培养. 印度梨形孢“DSM 11872”由长江大学张文英教授惠赠,其培养参照Johnson等[26]的方法进行. 大肠杆菌(Escherichia coli)感受态为DH5α菌株.
-
聚乙二醇(PEG-4000),马铃薯葡萄糖琼脂培养基(Potato Dextrose Agar,PDA),马铃薯葡萄糖肉汤培养基(Potato Dextrose Broth,PDB)等均购自北京酷来搏科技有限公司(Coolaber). 参照Johnson等[26]的方法配制改良PNM(modified PNM)培养基.
-
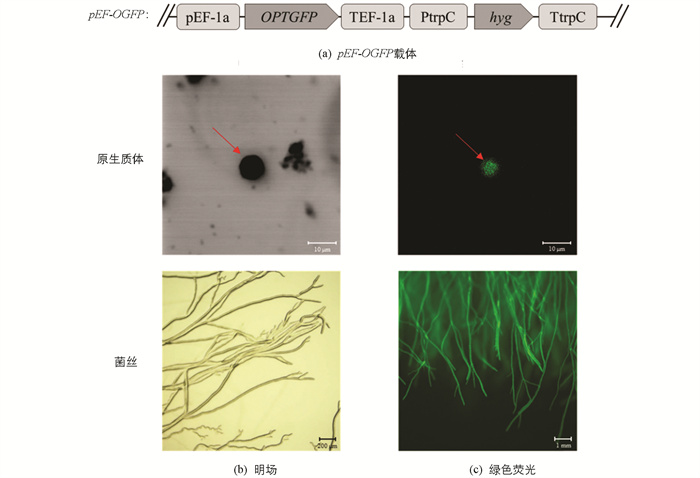
本研究用特异引物(表 1)扩增密码子优化的GFP(OGFP)基因序列[27],并将扩增产物OGFP片段融合于核盘菌EF-1a基因(SS1G_07595)启动子下游,获得pEF-OGFP过表达载体(图 1a). 参考Rollins[28]的方法进行原生质体制备及PEG介导的原生质体转化,将质粒载体转化至核盘菌“1980”基因组. 利用潮霉素筛选转化子,并在波长488 nm下观察转基因核盘菌的原生质体和菌丝,筛选“1980-GFP”菌株,用于后续试验.
-
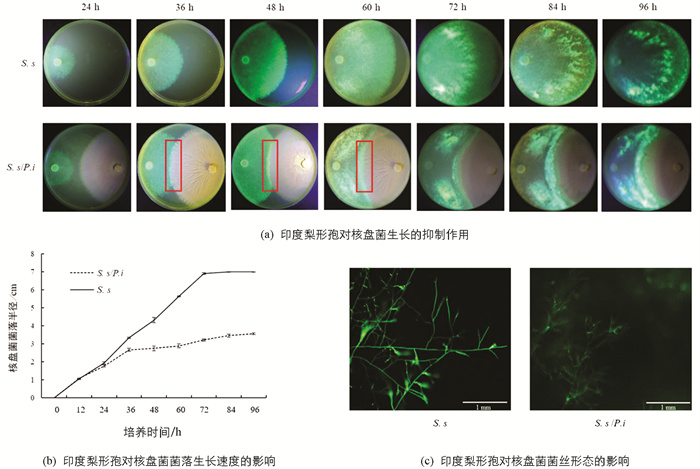
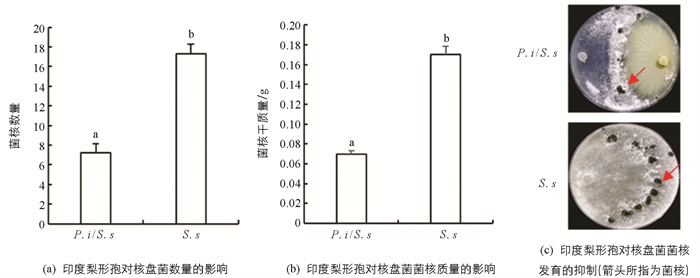
参照张斌等[29]的方法,进行平板对峙培养. 具体步骤:用无菌打孔器(直径6 mm)取印度梨形孢菌落边缘菌丝块,置于直径90 mm的PDA平板上,距平板边缘的1/8直径处. 待印度梨形孢菌落生长至平板2/3直径时,使用相同的方法取“1980-GFP”核盘菌的菌丝块置于平板的另一侧,以未放置印度梨形孢的平板为对照. 放置核盘菌后,每隔12 h拍照并测量菌丝块中心至菌落边缘的距离(cm),以评价菌丝的生长速度. 利用荧光体视显微镜观察两菌菌落的接触部分,并评价菌丝的形态特征. 分别于对峙培养后24 h,48 h,72 h及96 h取菌丝,立即置于液氮中保存,用于实时荧光定量PCR分析,3次重复. 对峙培养7 d后收集菌核,统计菌核数量及其干质量(g).
印度梨形孢发酵培养液培养核盘菌试验参考王慧俐[30]的方法进行,略有改动. 在无菌条件下,用无菌打孔器(6 mm)取5块印度梨形孢菌丝块置于PDB培养液中,于28 ℃,200 r/min摇床发酵培养. 培养4 d后,过滤并收集发酵培养液用于核盘菌培养,以PDB,1/2 PDB培养液培养核盘菌为对照. 22 ℃,200 r/min培养4 d后,比较各处理中核盘菌菌丝球的干质量(g),3次重复.
-
用试剂盒Ultrapure RNA Kit(DNase I)(北京康为世纪生物科技有限公司)进行总RNA提取与纯化;用试剂盒PrimeScript©RT reagent Kit(Perfect Real Time)(TaKaRa)进行cDNA反转录.
-
参照NCBI数据库中相关基因的编码序列,用Primer Premier 5设计引物(表 1). 反应体系:1 μL cDNA(100 ng/μL),SYBR Green Realtime PCR Master Mix 10 μL,正向及反向引物各0.5 μL,RNase-free H2O 8 μL. 用CFX96TM real-time PCR仪(Bio-Rad)进行实时荧光定量PCR检测. 反应程序:95 ℃预变性30 s,95 ℃变性5 s,60 ℃退火20 s,72 ℃延伸20 s,40个循环. 每个样本重复3次. 以核盘菌SsTubulin基因作为内参,按照2-ΔΔCT法计算基因的相对表达量[31].
-
用Microsoft Excel 2016和SAS 8.0软件进行数据分析,应用t测验法进行差异显著性检验.
1.1. 材料
1.1.1. 试验菌株
1.1.2. 试剂及培养基
1.2. 方法
1.2.1. pEF-OGFP载体构建与核盘菌原生质体转化
1.2.2. 核盘菌与印度梨形孢对峙培养
1.2.3. 总RNA提取及总cDNA第一链合成
1.2.4. 引物设计及实时荧光定量PCR
1.3. 数据分析
-
为更清晰地观察对峙培养下核盘菌的生长情况,本研究构建了绿色荧光蛋白的转基因核盘菌菌株. 利用PEG转化法,将密码子优化后的pEF-OGFP载体(图 1a)转入核盘菌菌株“1980”的原生质体中,经复壁培养和潮霉素抗性筛选,共获得26个转化子. 用激光扫描共聚焦显微镜和荧光体视显微镜观察原生质体和菌丝(图 1b,1c),筛选出绿色荧光信号最强的转化子,其菌落生长、菌丝形态以及菌核形成均与野生型一致,用于进行后续研究.
-
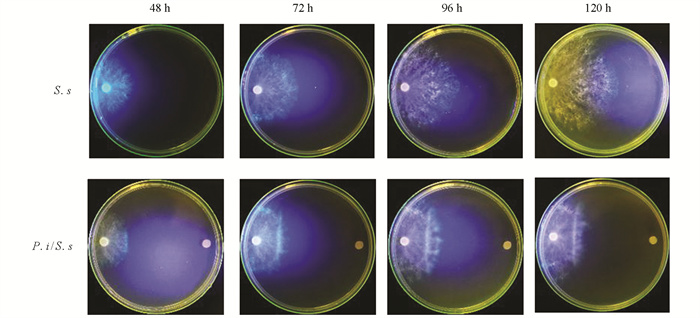
印度梨形孢与核盘菌菌落接触后,后者的生长受到显著抑制(图 2a). 在36 h时,其生长半径显著降低(p<0.05). 此后,处理组的菌落延伸半径降低了36.43%~53.56%,生长速度降低了84.76%(图 2b),并产生了抑菌带(图 2a红色方框). 抑菌带处核盘菌菌丝发育异常,其前端出现短而多的分叉,且呈弯曲生长,GFP信号也减弱(图 2c). 培养7 d后,对峙培养中的核盘菌菌核数量比对照降低了73.38%(图 3a),干质量降低了64.16%(图 3b). 印度梨形孢在PDA培养基上菌落生长致密,其菌丝可能会占据核盘菌的生长空间,进而抑制核盘菌生长. 印度梨形孢在改良PNM培养基上形成的菌落稀疏,并不会造成核盘菌生长空间受阻(图 4). 为此,本研究又在改良PNM培养基上进行印度梨形孢与核盘菌的对峙培养,结果发现核盘菌菌落生长仍然受到抑制(图 4),进一步表明印度梨形孢对核盘菌的抑制作用不仅仅源于生长空间竞争.
核盘菌菌丝块在液体培养基中会形成菌丝球. 利用不同的液体培养基培养核盘菌时,我们发现其菌丝球在PDB培养液中生长最旺盛,在1/2 PDB培养液中次之,但均比在印度梨形孢发酵培养液中的旺盛(图 5a). 将各处理的菌丝球烘干称量,发现在印度梨形孢发酵培养液中的核盘菌菌体干质量较PDB和1/2PDB培养液中均显著降低(p<0.05),降低量分别为52.01%和25.52%(图 5b).
-
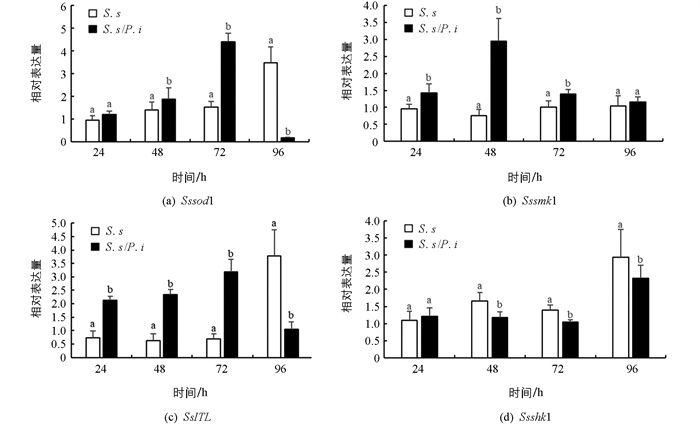
为研究对峙培养下印度梨形孢对核盘菌抑制的机理,我们用实时荧光定量PCR技术检测了与核盘菌菌落生长、菌核形成及逆境响应相关基因Sssod1,Sssmk1,Ssshk1及SsITL的相对表达水平.
Sssod1与核盘菌菌核萌发以及抗逆反应密切相关[32]. 在对峙培养早期,Sssod1显著上调(p<0.05),并于72 h达最高表达量,上调量为对照的2.78倍,随后表达量迅速降低,96 h时下调了94.53%(图 6a). 结果表明,印度梨形孢会诱导核盘菌的早期防御反应,在后期则会影响核盘菌菌核发育相关基因的表达,印证了对峙培养对核盘菌菌核数量和质量的抑制作用.
Sssmk1是核盘菌中信号传递的关键基因,同时与核盘菌菌落延伸、菌核发育相关[33]. 在对峙培养前期,Ssmk1表达量显著上调(p<0.05),48 h时上调3.89倍,后期恢复正常表达水平(图 6b). 结果表明,印度梨形孢影响了核盘菌的早期信号转导途径,激活了核盘菌逆境胁迫反应,并有可能加速了菌核的形成.
SsITL基因编码核盘菌的一个毒性分泌蛋白,对菌株的致病力、菌丝生长、菌核形成具有多效性[34]. 本试验中SsITL的表达与Sssod1极为类似,在对峙培养24~72 h内相对表达量显著上调(p<0.05). 上调量达2.94~4.71倍(图 6c). 印度梨形孢与核盘菌对峙培养过程中核盘菌菌落延伸受阻可能是该基因前期上调表达的原因之一,而后期该基因表达受到抑制则可能影响了核盘菌菌核的形成.
Ssshk1是一种组氨酸激酶,与核盘菌菌丝形态、菌落延伸以及对外界环境的适应力有关[35]. 在对峙培养初期,Ssshk1的表达水平与对照持平,中后期显著下调(p<0.05),下调量为20.81%~28.55%(图 6d). 结果表明,对峙培养过程中,印度梨形孢抑制了核盘菌Ssshk1基因的表达,可能导致了核盘菌菌丝形态异常,并在一定程度上影响了核盘菌对外界刺激的反应.
2.1. 绿色荧光蛋白转基因核盘菌菌株“1980-GFP”的获得
2.2. 印度梨形孢(P.i)对核盘菌(S.s)的抑制作用
2.3. 印度梨形孢对核盘菌生长发育与逆境响应关键基因表达水平的影响
-
对峙培养时,核盘菌与印度梨形孢接触部分产生了抑菌带,此后菌落延伸速度大幅下降,并在两菌接触边界形成气生菌丝,这与盾壳霉与核盘菌对峙培养时的现象类似[36]. 印度梨形孢与部分其他病原菌的对峙培养结果也与本研究类似. 印度梨形孢能够抑制灰霉病菌的生长[23];当在平板上优先培养印度梨形孢时能够抑制链格孢菌的生长[30];Ghahfarokihi等[37]用印度梨形孢与小麦全蚀病菌(Gaeumannomyces graminis var. tritici)对峙培养时,发现印度梨形孢与小麦全蚀病菌之间也会形成抑菌带,并且印度梨形孢会抑制小麦全蚀病菌菌丝的生长;印度梨形孢与黄萎病菌对峙培养时,发现其对黄萎病菌有直接的拮抗作用[21]. Moharam等[38]发现,平板对峙培养时,印度梨形孢并未对尖孢镰刀菌(Fusarium oxysporum)产生明显的拮抗作用,但其发酵培养液却能够显著抑制尖孢镰刀菌的生长. 印度梨形孢在生长过程中会产生种类繁多的次生代谢物,包括分泌蛋白、激素以及异羟肟酸(Hydroxamic acids)等[39-40]. 由异羟肟酸合成的衍生物N-甲酰羟胺BB-3497(N-formyl hydroxylamine BB-3497)是重要的抗菌剂,能够抑制部分病原真菌的生长[41];环异羟肟酸(Cyclic hydroxamic acid)能够增强植物对病原微生物的抗性[42]. 本研究收集了印度梨形孢的PDB培养液用以培养核盘菌,发现在印度梨形孢培养液中的菌丝球大小和干质量均显著小于以PDB和1/2PDB培养液培养的核盘菌菌丝球,表明印度梨形孢发酵培养液能抑制核盘菌的生长,但其具体抑制因子还有待进一步分析鉴定.
Sssod1是核盘菌超氧化物歧化酶(SOD)家族的重要成员[31],而Sssmk1通过核盘菌中信号传导,影响核盘菌菌落延伸、菌丝形态和菌核形成[32],二者均与核盘菌的防御反应与菌核形成密切相关. 本研究中Sssod1,Sssmk1在对峙培养前期表达均显著上调,表明对峙培养过程中,印度梨形孢激活了核盘菌的防御反应. SsITL编码核盘菌的一个毒性分泌蛋白,并与核盘菌菌核发育密切相关,在核盘菌侵染前期或生长受阻后迅速上调[33]. 本研究对峙试验表明,印度梨形孢阻碍了核盘菌的菌落延伸,SsITL在前期表达上调可能是与印度梨形孢接触后菌落延伸受阻所致. 然而,Sssod1与SsITL在对峙培养96 h时表达却受到抑制,印度梨形孢可能在对峙培养后期抑制了部分与核盘菌菌核发育相关基因的表达,对峙培养中核盘菌菌核数量和质量均较少也印证了该结果. 核盘菌菌核是由菌丝紧密交织而成的休眠体,能够帮助核盘菌抵御外界不良环境,利于核盘菌繁殖与继代[43],因此,印度梨形孢可能对核盘菌的繁殖以及抵御逆境的能力有抑制效果. Ssshk1调控核盘菌的应激反应,沉默表达Ssshk1的核盘菌菌丝形态异常,菌落延伸缓慢,并失去了对杀菌剂以及对外界胁迫的敏感性[34]. 本研究中,两菌接触后,核盘菌基因Ssshk1的表达有所降低,可能与对峙培养过程中核盘菌菌落延伸缓慢、菌丝形态异常密切相关. 对峙培养过程中,核盘菌基因Sssod1,Sssmk1,Ssshk1及SsITL的表达模式分析表明,印度梨形孢与核盘菌之间有着较为复杂的互作机制,有待进一步研究.
定殖了印度梨形孢的小麦对茎腐病的抗性显著提高,但对布氏白粉病菌的抗性并未提高[20];然而Molitor等[44]发现大麦在定殖印度梨形孢后,引起与大麦系统抗性相关基因的上调表达,调控大麦的抗氧化能力进而提高其对白粉病的抗性. 定殖印度梨形孢后,番茄植株的黄萎病严重程度降低了30%以上[45]. 本研究在体外证实了印度梨形孢对核盘菌有抑制作用,后续将验证印度梨形孢定殖于油菜后是否能够引起油菜菌核病抗性的改变,并深入探讨其相关机制.
-
印度梨形孢对核盘菌有直接抑制作用,能够抑制核盘菌菌落延伸,减少菌核数量与质量,并改变菌丝形态,且能够影响核盘菌防御反应及生长发育相关基因的表达.







 DownLoad:
DownLoad: