-
开放科学(资源服务)标识码(OSID):

-
胃癌是死亡率较高的常见癌症之一,在全球癌症死亡原因中排名第四[1-2]. 据2020年统计,全球每年胃癌新增病例109万人,死亡病例77万人[3]. 近年来,尽管胃癌发病率有所下降,但预计到2040年,这种恶性肿瘤的全球发病率将会增加62%[1, 4]. 癌症生物标志物的量化是控制和管理癌症最有效的策略之一[5]. 胃蛋白酶原(Pepsinogen,PG)是胃蛋白酶的无活性前体,根据其生化和免疫特性可以分为PG Ⅰ和PG Ⅱ两个亚群. 血清中PG Ⅰ、Ⅱ的水平以及它们的比值(PG Ⅰ/Ⅱ)被认为是胃癌筛查的有效生物标志物,其准确检测可以有效地对患者的胃癌患病风险进行分级,并决定进一步的检查策略[1, 6]. 传统的蛋白检测方法如高效液相色谱法[7-8]、酶联免疫吸附测定法[9]、气相色谱法[10]等可以提供精确的检测结果,但这些方法操作复杂、费时费力,难以满足大规模筛查的即时检测需求. 因此,针对胃癌的早期筛查和治疗控制建立快速准确的特异性检测方法具有重要意义.
侧流免疫层析(Lateral Flow Immunoassay,LFIA)是一种经典的即时检测技术,因其简单、快速、便宜等优势,已被广泛应用于早期疾病诊断、食品安全检测和环境监测等领域[11-13]. 然而,基于胶体金的传统LFIA的灵敏度较低,定量检测困难,大多用于定性或半定量检测. 为了提高LFIA的灵敏度,各种纳米颗粒被开发作为探针来增强信号,如荧光、上转换、磁性和光热纳米材料等[12]. 尽管这些纳米颗粒的引入使得LFIA可与先进的检测技术相媲美,但它们都只能提供单一维度的信息,难以满足用户在不同场景中的检测需求. 例如,时间分辨荧光LFIA的信号读取仪器价格昂贵,在资源有限的地区普及受限[14];磁性纳米材料能够有效地从复杂的基质中富集目标物从而提高检测灵敏度,但磁信号很容易受到外部环境的干扰,这无疑增加了对实验环境的要求[15-16]. 因此,有必要针对不同的诊断场景开发具有多维信号(多模态)输出的多功能纳米探针.
LFIA典型的输出检测信号包括比色、荧光、磁、光热、拉曼等[11-12, 17-22]. 其中,比色信号具有视觉定性解释的便捷性,是LFIA最优秀的特征之一[12, 23]. 相对于比色信号来说,荧光信号的灵敏度较高,是定量分析最常见的选择[11-12]. 由于磁信号的低背景噪声,它被认为有潜力实现比荧光信号更高的灵敏度,此外,磁性探针能够提供富集和分离功能[15-16],但需要特殊的设备支持[21]. 光热信号具有高灵敏度,可以通过手持式红外相机或普通温度计获得[19, 24-25],并且由于光热效应能够实现精确的热量控制,因此在治疗应用、抗菌领域引起了巨大的研究兴趣[26-27]. 表面增强拉曼散射则因为其激发波长较长,可以有效避免荧光猝灭和光漂白的干扰,可实现超灵敏的检测,被认为是一种极具潜力的技术[28-29]. 多模态LFIA即是利用多功能纳米探针实现各种信号之间的组合,不仅能够保持定性模式下便捷的视觉解释,而且还集成了不同灵敏度水平的各种定量模式以适应不同的应用场景,在即时检测的实用性和灵活性方面有巨大提升,已迅速发展成为一个新兴的研究和开发方向[30-32]. 例如,Huang等[32]将磁性材料和荧光团嵌入纳米结构中制备了一种双功能的纳米探针,结合磁性免疫分离技术和免疫荧光检测技术,无需洗脱和孵育步骤即可灵敏检测食源性病原体,但该研究没有将磁信号作为输出来进一步增加检测的弹性. Hu等[17]开发了一种比色—荧光—磁性纳米球多模态检测平台,实现了靶标分离富集、多信号读出和两种定量检测的多功能集成. Li等[24]利用自组装的多价荧光纳米抗体和金属有机碳纳米材料构建的比色、荧光和光热多模态探针,在黄曲霉毒素B1的检测中表现出优异的性能.
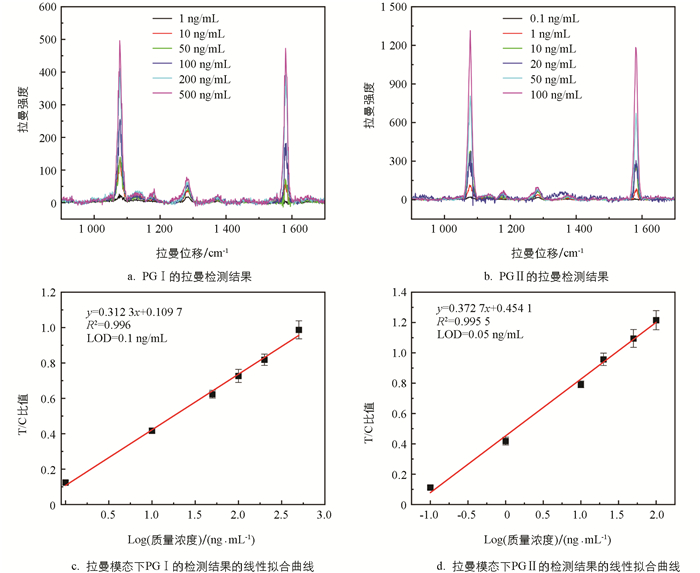
本研究通过金包裹超顺磁性纳米晶簇(SMNC@Au)合成了一种集成比色、磁和拉曼信号的多功能纳米探针,实现了对胃癌标志物的多模态检测. 探针SMNC@Au是首先通过水热法合成的超顺磁性纳米晶簇作为核心,然后通过种子生长法在其上涂覆一层金壳,并在金壳表面结合4-巯基苯甲酸(4-MBA)作为拉曼报告分子而形成的. 该多功能探针可以提供比色、磁性和拉曼3种信号,且可以通过磁力实现分析物的分离和富集,极大简化样本的处理. 最终,基于SMNC@Au的LFIA检测平台能够实现PG Ⅰ和PG Ⅱ的同时检测,并具有一种视觉定性模式和两种不同灵敏度的定量模式(图 1). 为了证明其可行性,本研究使用了不同质量浓度的样本组进行了性能验证. 结果表明,PG Ⅰ和PG Ⅱ的视觉检测限分别为10 ng/mL和1 ng/mL. 在磁性模式下,可以将PG Ⅰ和PG Ⅱ的检测限分别降低到0.5 ng/mL和0.1 ng/mL. 而拉曼模式可以大幅提高PG Ⅰ和PG Ⅱ检测的灵敏度,其检测限分别为0.1 ng/mL和0.05 ng/mL. 磁性模式和拉曼模式可以分别实现从1 ng/mL到500 ng/mL(PG Ⅰ)和0.1 ng/mL到100 ng/mL(PG Ⅱ)的检测范围,并具有高准确性,完全符合临床诊断标准. 此外,PG Ⅰ和PG Ⅱ之间没有内源性交叉反应. 基于金包覆磁性纳米探针的多模态LFIA能够显著提高即时检测的灵活性和普遍适用性,在LFIA领域具有巨大的发展潜力.
HTML
-
无水氯化铁(FeCl3)、乙酸钠、柠檬酸三钠二水合物(Na3Cit)、盐酸羟胺、甘氨酸(GLY)、氯金酸(HAuCl4)、4-巯基苯甲酸(4-MBA)购自上海麦克林生化科技有限公司. 乙二醇(EG)、硼氢化钠(NaBH4)、乙醇胺(EA)、2-(N-吗啉)-乙烷磺酸(MES)、牛血清白蛋白(BSA)购自上海阿拉丁生化科技股份有限公司. 乙基-3-(3-二甲基氨基丙基)-碳二亚胺(EDC)、N-羟基琥珀酰亚胺(NHS)和聚乙烯亚胺(PEI)溶液购自Sigma-Aldrich(上海,中国). 玻璃纤维、吸水垫和基板购自上海金标生物技术有限公司. 硝酸纤维素(NC)膜购自赛多利斯有限公司. 磷酸盐缓冲液(PBS)购自长春赛斯医疗生物工程有限公司. 聚乙烯吡咯烷酮(PVP)购自Diamond生物技术有限公司. 抗人PG Ⅰ mAb K56t1(Anti-PG Ⅰ-D)、抗人PG Ⅰ mAb K50f4(Anti-PG Ⅰ-C)、抗人PG Ⅱ mAb K30t2(Anti-PG Ⅱ-D)、抗人PG Ⅱ mAb K28c9(Anti-PG Ⅱ-C)以及标准品PG Ⅰ mAb、PG Ⅱ mAb均购自南京欧凯生物科技有限公司. 兔IgG(R-IgG)和山羊抗兔IgG(G-IgG)购自山东亚瑞特生物科技有限公司. 所有实验均使用去离子水.
SMNC和SMNC@Au的形态和元素分布使用TESCAN扫描电子显微镜(SEM)MIRA3表征. 动态光散射(DLS)设备用于表征纳米颗粒的粒径、Zeta电势和聚合物分散性指数(PDI). 超声波均质器SCIENTZ-IID(购自宁波赛特生物科技有限公司)用于分散颗粒. LakeShore7404振动样品磁强计(VSM)用于表征SMNC和SMNC@Au的饱和磁化强度. 拉曼分析仪(购自法国侯布里亚公司)用于检测拉曼信号. 基于本团队之前使用的微型磁检测设备[22]检测磁信号. 划膜喷金仪(HM3035)用于在NC膜上固定抗体. 测试条切割机(WM-100)用于生成侧流免疫层析试纸条. 电热鼓风炉用于干燥玻璃纤维和PVC基板.
-
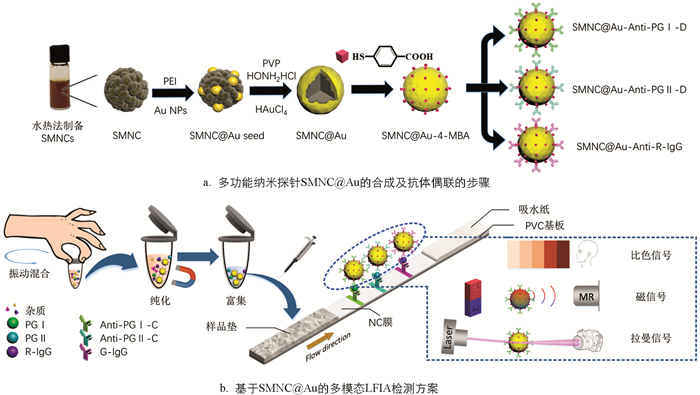
图 1a展示了核壳结构的多功能纳米探针SMNC@Au的合成过程. 根据文献[33],使用水热法合成了超顺磁性纳米晶簇(SMNC)来作为探针的核心. 首先向20 mL的乙二醇中加入649 mg无水FeCl3和200 mg Na3Cit,充分搅拌后,添加2 g乙酸钠继续搅拌30 min. 随后,将液体倒入50 mL的高温反应釜中,在205 ℃的条件下反应12 h. 最后,所得液体用去离子水和乙醇磁吸洗涤5次,得到粒径均匀的SMNC颗粒(约190 nm).
金壳的制备采用种子原位生长法[34]. 首先将10 mg的SMNC颗粒分散于1 mL的去离子水中,并加入110 μL 10 mg/mL的PEI溶液,超声处理20 min,得到SMNC@PEI溶液. 将1 mL 10 mg/mL的SMNC@PEI溶液加入到6 mL提前制备的Au溶液[35]中,超声处理30 min后,用去离子水磁吸清洗得到SMNC@Au种子颗粒. 随后将1 mg的SMNC@Au种子分散在10 mL 3 mg/mL PVP和0.5 mg/mL盐酸羟胺溶液中超声处理15 min,并在超声过程中缓慢滴入10 μL 50 mmol/L HAuCl4溶液,继续超声处理15 min. 经过两次去离子水洗涤后得到SMNC@Au. 采用4-MBA作为表面修饰拉曼报告分子. 将1 mg SMNC@Au溶于2 mL 10 μmol/L 4-MBA乙醇溶液中,振动过夜. 然后用乙醇洗涤两次后分散在乙醇中备用.
-
准备1mg SMNC@Au-4MBA探针,用pH值为6.5的MES缓冲液洗涤上述探针两次并最终分散于1 mL的MES缓冲液中. 在缓冲液中加入1.2 mg NHS和1.5 mg EDC并振动1 h以活化羧基. 然后利用磁铁将反应完的探针析出并分散于1mL 1x PBS溶液中. 100 μL抗体(Anti-PG Ⅰ-D、Anti-PG Ⅱ-D或R-IgG)加入到溶液中,反应4 h用于偶联抗体. 再将100 μL 10% BSA溶液、200 mg/mL甘氨酸溶液和200 mmol/L乙醇胺溶液加入到上述溶液中,继续振动1 h. 随后用磁铁将探针标记后的抗体析出并用PBS清洗3次,最后存放于1%BSA溶液中备用.
-
免疫层析试纸条由PVC基板、玻璃纤维(样品垫)、NC膜和吸水纸组成,如图 1b所示. 所用的玻璃纤维在由1.21%的Tris、1%的PVP、1%的S9、5%的T-酪蛋白组成的处理液进行浸泡,然后放置于37 ℃的鼓风干燥箱干燥4 h. 利用划膜喷金仪将0.8 mg/cm的Anti-PG Ⅰ-C、Anti-PG Ⅱ-C和G-IgG固定在NC膜上,分别形成两条测试线(T1和T2)和质控线(C). 然后将试验卡片放置于37 ℃烘箱中过夜干燥. 最后,使用切割机将试验卡片裁切成宽度为4.05 mm的小条并装配卡壳,干燥保存.
-
图 1b展示了基于SMNC@Au的多模态LFIA的检测方案. 首先将10 μL样品滴加到试管中,然后分别滴加质量浓度为2 mg/mL的SMNC@Au-Anti-PG Ⅰ-C、4 mg/mL的SMNC@Au-Anti-PG Ⅰ-C和1.5 mg/mL的SMNC@Au-R-IgG各2 μL,混合大约1 min后,使用磁铁富集颗粒并用移液枪吸出原有液体. 随后将样品用100 μL 1x PBS稀释,然后滴加到样品垫上. 15 min后,即可进行比色信号的观察. 20 min后,可用自制的手持设备进行磁性模式检测. 在排除残余水分干扰后,可以在拉曼信号检测仪下对表面增强拉曼散射(SERS)信号进行测量.
1.1. 主要材料和仪器
1.2. 多功能纳米粒子的制备
1.3. 抗体偶联
1.4. 免疫层析试纸条的组装
1.5. 检测流程
-
LFIA是基于抗原与抗体的特异性结合实现对特定目标分子的识别和捕获. 可根据分子量的大小,使用夹心法或竞争法来检测目标分子. 在本研究中,由于PG Ⅰ和PG Ⅱ的分子量较大,故采用更为合适的夹心法. 在预处理样品时,探针标记的抗体会先和目标结合形成复合物. 当预制备的样品溶液被滴加到样品垫上后,液体将在毛细管力的作用下沿NC膜流向吸水垫. 在此过程中,样品中包含有PG Ⅰ、PG Ⅱ抗原的复合物将分别被固定在两个测试线(T1与T2)上的Anti-PG Ⅰ-C和Anti-PG Ⅱ-C捕获,形成夹心结构. 不管样品中是否存在PG Ⅰ和PG Ⅱ,探针标记的R-IgG都将被固定在质控线上的G-IgG捕获. 层析完成后,可以通过观察NC膜上的线条颜色或检测磁信号或SERS信号的强度来实现目标分子浓度的检测. 值得注意的是,本研究的试纸条与传统的免疫层析试纸条的结构不同,通过混合样品和探针进行预处理,不需要使用结合垫,从而精简了试条的结构. 此外,本研究利用探针SMNC@Au磁性在外部磁场下对样品进行富集和清洗,这极大地提高了检测灵敏度. 同时,它也为自动化样品预处理系统的集成研发提供了一个思路.
-
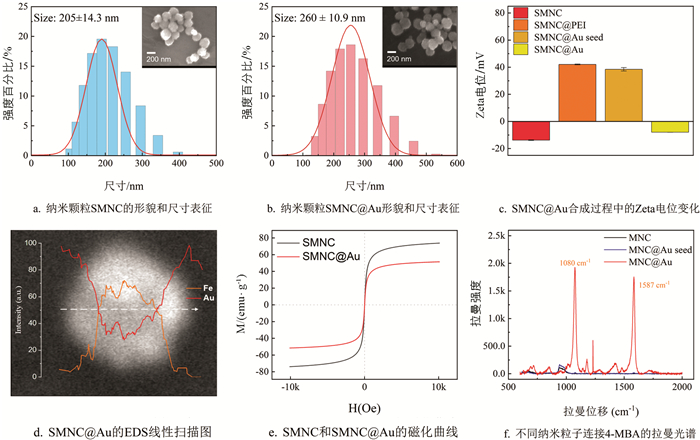
水热法合成的磁性纳米颗粒的粒径通常为180~300 nm. 相比于化学共沉淀法,水热法合成的颗粒在磁性、分散性和比表面积方面表现更优. 因此,我们采用水热法来合成SMNC作为探针颗粒的核心. 所合成的SMNC由粒径非常小的Fe3O4纳米晶体组成,这不仅使SMNC具有超顺磁性,而且有利于金壳的涂覆和抗体的结合. 在其他条件不变的情况下,调整柠檬酸钠的量可以调整合成的SMNC粒径大小[33],SMNC的饱和磁化强度随着SMNC粒径的增大而增加. SMNC的饱和磁化越强,操作起来越容易,产生的信号越强. 但过大的粒径可能会在免疫层析过程中引起堵塞并影响LFIA的结果. 使用扫描电子显微镜(SEM)和动态光散射(DLS)对合成的SMNC形貌和尺寸进行表征,结果如图 2a所示. 可以看到,本研究合成的SMNC颗粒的平均粒径为205 nm,PDI为0.018,尺寸分布均匀,金壳的合成采用种子原位生长法. 图 2c显示了在金壳涂覆过程中纳米颗粒的Zeta电位变化. 首先,通过超声波将PEI涂覆在Fe3O4表面. 由于PEI中含有大量的胺基团,与SMNC相比,SMNC@PEI的Zeta电位急剧增加. 随后,添加金纳米颗粒溶液以提供Au种子. 金纳米颗粒带负电,通过静电吸附在SMNC周围. 此时,由于仍然有很多胺基团暴露出来,纳米颗粒的电位只是略微降低. 为了得到完整的金壳,以SMNC周围吸附的金颗粒为种子,加入HAuCl4还原为金,使Au种子不断生长,最终在SMNC表面形成了一层不均匀的金壳. 此时纳米颗粒SMNC@Au上的胺基被金壳完全包围,Zeta电位大幅降低. 图 2b中显示了SMNC@Au颗粒的形貌和大小,其中的插图为SEM图像. 此外,对单个SMNC@Au进行了EDS线性扫描(图 2d),可以发现金元素的相对强度在颗粒边缘较高,在中心较低,而铁元素的分布与金恰恰相反,这证明金已成功涂覆在SMNC上. 最后,测试了SMNC和SMNC@Au的磁化曲线(图 2e),结果显示合成的颗粒无剩磁,饱和磁化强度分别达到73.9 emu/g和51.5 emu/g. 这表明本研究合成的探针保持了高饱和磁化强度.
产生独特且强大的拉曼信号需要两个条件:报告分子和合适的基底. 过去的研究表明,不均匀的胶体金表面可以显著增强拉曼信号. 本研究选择4-MBA作为拉曼报告分子,因为它具有3个优势:一是4-MBA含有硫醇基团,可以轻易地与金壳反应形成金-硫键,使4-MBA能稳定地绑定在SMNC@Au表面;二是4-MBA的苯环振动可以产生独特的拉曼信号,且能被胶体金增强;三是4-MBA的生物相容性强,它含有暴露的羧基,激活后可以轻易地与蛋白质的胺基团结合. 为了证明SMNC@Au的SERS效应,本研究分别将0.5 mg的SMNC、SMNC@Au seed和SMNC@Au分散在1 mL的10 μmol/L 4-MBA溶液中,混合均匀后,用乙醇洗涤纳米颗粒3次. 然后测试了它们的拉曼信号,结果如图 2f所示. 4-MBA标记的SMNC@Au颗粒在4-MBA特征峰处(1 080 cm-1和1 587 cm-1)显示出强烈的信号,证明了其显著的增强作用. 相比之下,SMNC颗粒不能与4-MBA反应,在清洗后表面没有报告分子附着,因此无法检测到4-MBA的特征峰. SMNC@Au种子表面吸附了少量的金纳米颗粒,可以与4-MBA结合,因此在拉曼信号光谱中可以观察到弱的特征峰.
-
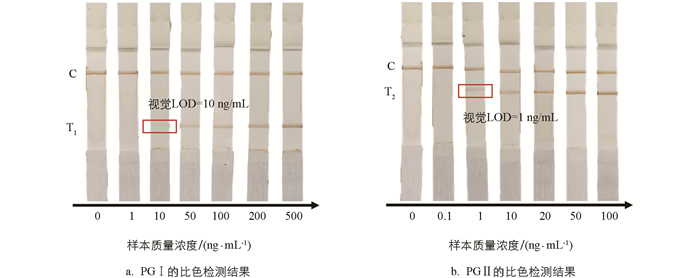
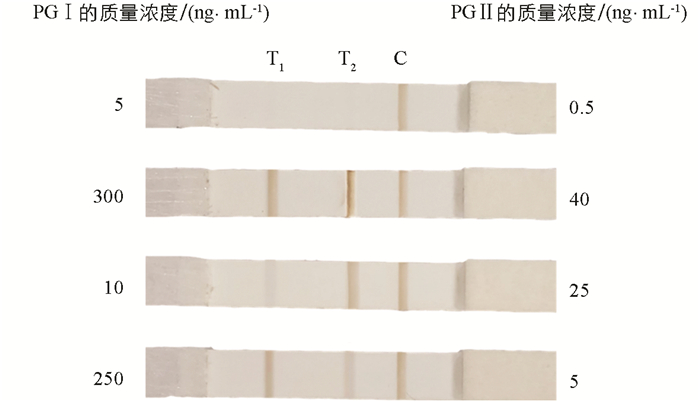
为了验证本研究开发的比色—磁—拉曼多模态LFIA系统的性能,使用一系列不同质量浓度的PG Ⅰ和PG Ⅱ的溶液样本进行测试. 根据人血清中PG Ⅰ和PG Ⅱ的质量浓度范围(PG Ⅰ:32.69~129.35 ng/mL;PG Ⅱ:3.51~20.68 ng/mL)[36-39],分别设置了6个不同质量浓度的PG Ⅰ(1,10,50,100,200,500 ng/mL)和PG Ⅱ(0.1,1,10,20,50,100 ng/mL)的溶液样本,每个样本测试3次. 图 3显示了层析的比色检测结果. 可以看到随着样本质量浓度的增加,T线的颜色逐渐变深,而C线的颜色基本保持不变. 通过肉眼观察得到PG Ⅰ和PG Ⅱ的视觉检测限分别为10 ng/mL和1 ng/mL.
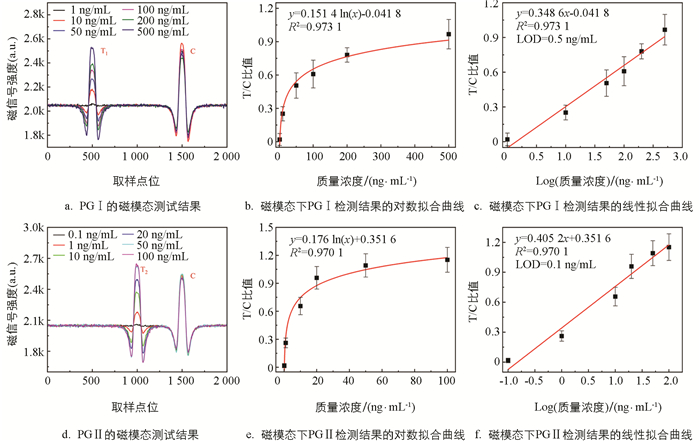
对于磁信号的检测,采用课题组之前研究自制的微型检测仪[22]进行分析,该仪器能够自动扫描试纸条上每点的磁强度,从样本到结果整个检测时间低于20 min. 记录不同质量浓度的PG Ⅰ和PG Ⅱ样本的磁模态信号,如图 4a和4d所示. 在T线和C线的位置可以观察到明显的磁信号峰,C线处的磁信号强度几乎不变,而T线的信号强度随着样本质量浓度的降低而减小,当样本质量浓度过低时,磁信号几乎淹没在噪声中. 以样本质量浓度为X轴,T/C值为Y轴对其进行了拟合分析,结果如图 4b和4e所示,两者呈对数关系. 对质量浓度进行对数变换,得到了质量浓度和T/C值之间的线性拟合曲线(图 4c和4f),其相关系数R2分别为0.973 1(PG Ⅰ)和0.970 1(PG Ⅱ),表明两者高度相关. 目前,一般将检测限(LOD)定义为信噪比3∶1时的质量浓度. 因此,本研究使用空白样本评估了磁模态检测下系统的LOD,PG Ⅰ和PG Ⅱ的LOD分别为0.5 ng/mL和0.1 ng/mL.
为了准确地检测SERS信号,本研究沿试纸条流动方向均匀选取3个区域进行拉曼映射,每个区域包含52个均匀分布的点. 以这3个区域获得的1 587 cm-1处的拉曼信号的平均值作为有效测量信号来评估拉曼模态的检测性能. 图 5显示了拉曼模式下的测试结果. 可以看到,相比于磁模态,拉曼检测的性能表现更好. PG Ⅰ和PG Ⅱ的线性相关系数R2分别为0.996 0和0.995 5,LOD分别到达了0.1 ng/mL和0.05 ng/mL. 值得注意的是,拉曼模式获得准确检测结果耗时更长,大约需要3 h,而磁信号测试只需约20 min.
此外,使用特定质量浓度的样本来验证检测的准确性以及两种抗原之间是否存在内源性交叉反应,每个样本测试5次. 比色结果(图 6)显示,两条T线上的颜色深度与样品质量浓度呈正相关,这证明了PG Ⅰ和PG Ⅱ之间没有内源性交叉反应. 本研究统计了两种定量模式的重复性和准确度数据,详细统计结果如表 1所示. 磁模式下,PG Ⅰ和PG Ⅱ样本5次重复测试的变异系数(CV)分别为8.4%~18.7%和11.4%~21.0%,准确度分别为80.3%~108.6%和80.6%~110.1%. 而拉曼模式下,PG Ⅰ和PG Ⅱ样本5次重复测试的变异系数分别为3.4%~6.8%和3.2%~13.2%,准确度分别为97.7%~104.5%和95.6%~106.0%.
表 2列出了本研究方法和一些其他免疫层析方法的性能比较. 与其他免疫层析方法相比,磁模式在相近的时间内实现了更优的检测性能,同时,本研究的仪器操作简单且造价便宜,在社区筛查和家庭自测的应用环境中具有巨大的潜力. 而拉曼模式的检测精度可以媲美实验室方法,从而满足医院诊断的更高要求.
2.1. 检测原理
2.2. 多模态探针的表征与优化
2.3. 多模态LFIA系统检测性能
-
本研究成功合成了一种具有磁性和表面增强拉曼散射(SERS)效应的多功能纳米探针,并基于此探针开发了用于同时检测胃癌标志物PG Ⅰ和PG Ⅱ的多模态LFIA. 该检测平台可以同时有效地输出比色、磁和拉曼三重信号,并同时提供两种目标分子的一个定性和两个定量的检测结果,允许用户根据不同的测试条件灵活选择应用模式,以适应不同的场景和要求. 上述研究结果表明,这种多模态检测方法为侧流免疫层析检测提供了更加灵活高效的平台.






 DownLoad:
DownLoad: