-
开放科学(资源服务)标识码(OSID):

-
烟草(Nicotiana tabacum L.)是我国重要经济作物,由茄科劳尔氏菌(Ralstonia solanacearum)引起的烟草青枯病和烟草疫霉(Phytophthora nicotianae)引起的烟草黑胫病是烟叶生产上的两种主要土传病害[1-2]。两种病害常复合侵染烟株[3-4],严重时发病率高达75%[5],在连作和高温高湿环境下,产量损失可达100%[6]。长期连作导致病原菌耐药性逐年增强,病害复合侵染后更难防控[7-8]。
了解病原菌生物学特性是研究病害灾变规律的基础。烟草疫霉在21~35 ℃的条件下生长良好且具有致病性[9],30 ℃时生长速度较快,35 ℃时致病性较强;在28~32 ℃条件下宜产生孢子及萌发菌丝。进一步研究显示,菌株在30 ℃时具有广泛的营养需求特性、渗透压和pH适应能力,同时具有较强的致病性[10]。茄科劳尔氏菌在低于20 ℃环境中无致病性,30~35 ℃条件下致病性最强,繁殖效率最高[11],对35 ℃时的渗透压和pH适应能力高于20~24 ℃时[12]。以上均为单一病原菌生物学特性,在烟叶生长中后期,两种病害常混合发生,存在复合侵染的情况,但两种病原菌共存时,烟草疫霉的生物学特性变化规律尚不明确。
碳源是影响植物病原菌生存和致病性的主要营养物质[13],病害发生后,其病际微生物的碳源代谢功能可反映植物健康情况。Biolog ECO微平板法可快速检测微生物群落对不同碳源的代谢值,反映不同环境下微生物群落代谢差异特征[14],目前尚不清楚当烟草青枯病与烟草黑胫病复合侵染时,其烟叶病际微生物群落间的代谢功能变化。
本文测定了茄科劳尔氏菌和烟草疫霉共存时烟草疫霉的生物学特性及致病性,并通过Biolog ECO代谢表型技术测定其病际微生物的碳源代谢值,旨在探究两种病害复合侵染规律,为烟草黑胫病和青枯病的联合防控提供参考依据。
HTML
-
茄科劳尔氏菌RS#9和烟草疫霉菌HJ7均由本试验室前期分离鉴定获得。试验烟草品种为云烟85,由贵州省烟草科学研究院提供。
-
酪蛋白氨基酸肽葡萄糖培养基(CPG):酪蛋白水解物1.0 g,蛋白胨10 g,蔗糖5.0 g。
半选择性培养基(SMSA):蛋白胨10 g,甘油5.0 g,酪蛋白水解物1.0 g,琼脂15 g,高压灭菌后的溶液中加入1%结晶紫试剂5 mL,2%TTC试剂2.5 mL,杆菌肽25 mg,多粘菌素B 100 mg,氯霉素5 mg,氨苄青霉素0.5 mg。
利马豆培养基(LBA)[15]:利玛豆60 g/L煮沸1 h并过滤,滤液中加入15 g琼脂,不加琼脂时即LB培养基。
10% V8培养基:900 mL无菌水中加入100 mL坎贝尔V8果汁和0.2 g碳酸钙。
以上培养基中各组分均为配制1 000 mL培养基用量,pH为自然值。
-
Biolog Omnilog全自动微生物鉴定系统和Biolog ECO碳源代谢板(No.1506),购自美国Biolog公司。
-
将烟草疫霉菌HJ7接种于含250 μg/mL氨苄青霉素的LBA平板上,于28 ℃黑暗条件下预培养7 d。将茄科劳尔氏菌菌株RS#9在SMSA培养基上划线培养,挑取单菌落置于盛有50 mL CPG培养基的三角瓶中,28 ℃、200 r/min摇培12 h,菌液浓度达到2×109 cfu/mL时备用。在烟草疫霉菌落外缘打取直径6 mm的菌饼接种于预先涂布30 μL/皿茄科劳尔氏菌菌液的LBA培养基上(共培养处理组),以只接种菌饼的LBA平板作为烟草疫霉对照组,每处理重复3次。接种后1~5 d分别通过“十字交叉”法测量烟草疫霉菌落直径,计算菌丝生长速率[16]。
-
在培养7 d的HJ7菌落外周制备直径6 mm的菌饼,将其置于盛有50 mL LB培养基的三角瓶中,每瓶10个菌饼,接菌后向各三角瓶里添加200 μL浓度为2×109 cfu/mL的RS#9菌液(共培养处理组),以不添加菌液的处理作烟草疫霉对照组,各6次重复。接菌后,将三角瓶置于28 ℃、180 r/min的摇床中培养,分别培养1 d、3 d、5 d、7 d、10 d后在无菌条件下过滤菌丝并称取菌丝鲜重,计算各处理组的菌丝鲜重增量。烘干后称取菌丝干重,比较不同处理的干重差异。
-
在28 ℃培养7 d的HJ7菌落外周打取10个直径6 mm的菌饼,放入含20 mL液体V8培养基的培养皿中,加入200 μL浓度为2×109 cfu/mL的RS#9菌液(共培养处理组),以不添加菌液的处理作为烟草疫霉对照组,各6次重复。在28 ℃黑暗条件下培养72 h后持续光照3 d诱导产生孢子囊[17-18],待菌丝上产生大量孢子囊后,两组各挑取适量菌丝置于显微镜下利用血球计数板测定单位面积(mm2)的孢子囊数量。除去培养皿中的液体,重新加入10 mL无菌水,置于4 ℃冰箱30 min后转至25 ℃培养箱中全黑暗培养约2 h,刺激孢子囊释放游动孢子,显微镜观察游动孢子释放情况。
-
在28 ℃黑暗培养7 d的HJ7菌落外围打取10个直径6 mm的菌饼,其中一份与茄科劳尔氏菌共培养后备用。采集云烟85健康烟苗(6~7叶期)第3~4叶位叶片,用无菌水冲洗2次后晾干,在叶片主脉两侧选择两个点位接种[19]。以接种LBA培养基的刺伤叶片为空白对照组,将直径6 mm菌饼接种于刺伤烟叶上作为烟草疫霉对照组,以接种共培养后烟草疫霉菌饼的烟叶作为共培养处理组,每个处理6次重复。接菌后,将叶片置于30 ℃光照培养箱中(L/D:12 h/12 h,RH:90%±2%,光照强度:6000 Lux)。培养1 d、3 d、5 d后,采用“十字交叉法”分别测量各处理的病斑直径。
观测结束后,用消毒剪刀剪取5 g空白对照组刺伤部位叶片及各处理组病际组织叶片,放在盛有50 mL 0.8%的无菌生理盐水的100 mL三角瓶中,28 ℃、180 r/min摇培2 h,静置30 min后吸取上清液依次加入Biolog ECO代谢板微孔中,每孔100 μL。将接菌后的ECO代谢板置于Omnilog恒温培养箱,28 ℃培养7 d,采用Biolog仪器专用软件收集病际微生物碳源代谢的颜色值[20],使用HemI软件制作热图。
-
使用Excel 2019、DPS 2019、Sigma Plot、HemI等软件进行数据处理与统计分析。
1.1. 材料
1.1.1. 供试菌株与品种
1.1.2. 供试培养基
1.1.3. 鉴定系统和代谢板
1.2. 生物学习性
1.2.1. 烟草疫霉菌丝生长速率
1.2.2. 烟草疫霉菌丝生物量
1.2.3. 烟草疫霉孢子囊数量及游动孢子释放情况
1.3. 致病性及病际微生物碳源代谢功能
1.4. 数据处理及统计分析
-
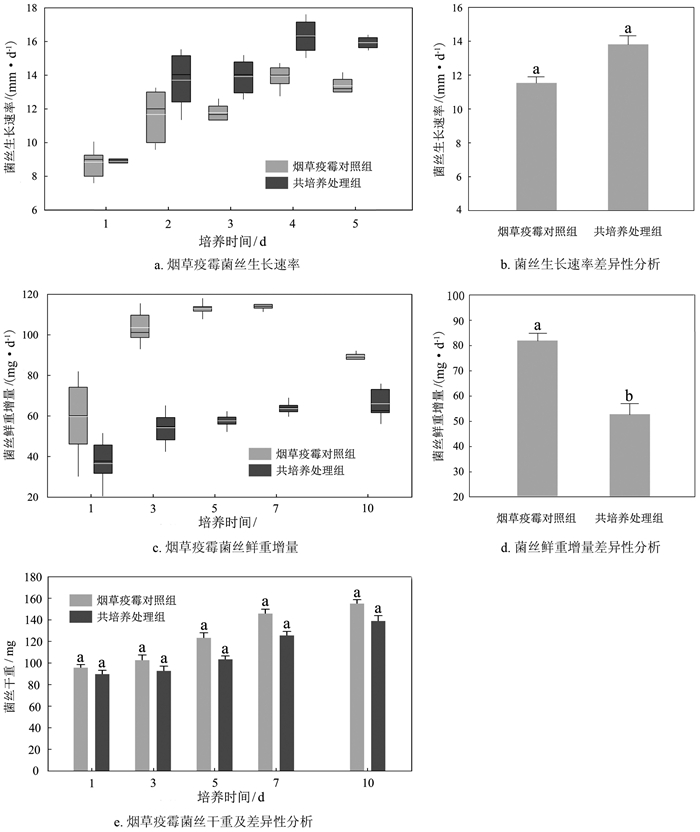
烟草疫霉对照组菌丝生长速率平均为11.92 mm/d,第4 d生长速率最快,达到14.16 mm/d;共培养处理组菌丝生长速率平均为13.71 mm/d,第4 d生长速率最快,达到17.09 mm/d(图 1a)。如图 1b所示,组间不存在显著性差异(p>0.05)。
摇培时,烟草疫霉对照组菌丝鲜重在第1~7 d内平均增长82.08 mg/d,共培养处理组菌丝鲜重在第1~7 d内平均增长52.29 mg/d(图 1c),共培养处理组的菌丝鲜重增量显著低于对照组(图 1d)。培养期间,烟草疫霉对照组菌丝干重平均增加58 mg/d,共培养处理组增加49 mg/d,组间不存在显著性差异(图 1e)。
-
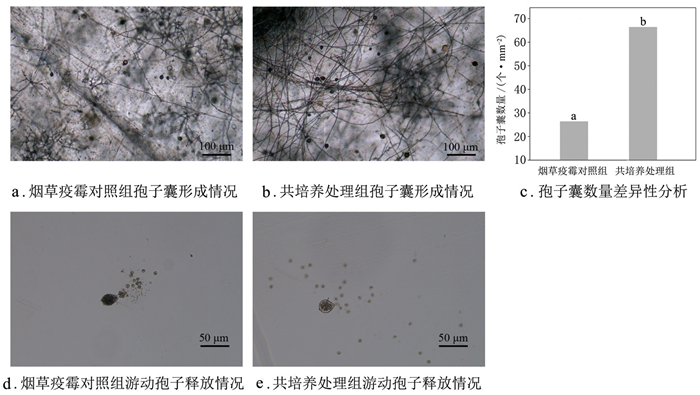
孢子囊形成情况如图 2a、图 2b所示,共培养处理组孢子囊数量平均为64.6个/mm2,烟草疫霉对照组平均值为28.6个/mm2,显著低于共培养处理组(图 2c)。共培养处理不影响烟草疫霉孢子囊正常释放游动孢子,烟草疫霉对照组(图 2d)和共培养处理组(图 2e)的游动孢子均能正常形成休止孢。
-
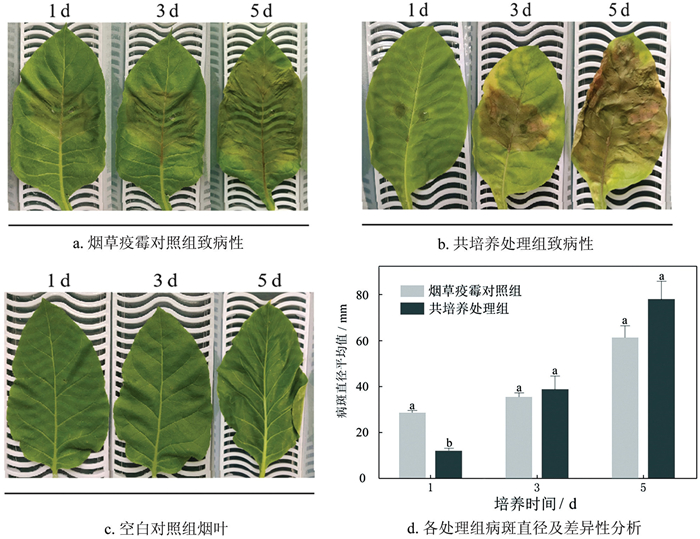
致病性结果(图 3a、图 3b)表明:第1 d时,烟草疫霉对照组与共培养处理组间病斑直径存在显著差异(图 3d)。第3 d时,烟草疫霉对照组与共培养处理组的病斑直径不存在显著性差异,共培养处理组病斑周围黄化,病斑中心穿孔。第5 d时,烟草疫霉对照组病斑面积增幅较小,病斑直径由第1 d的27.25 mm增至61.67 mm;共培养处理组病斑周围黄化面积扩大至整叶,叶片萎蔫,病斑直径增至78.46 mm。
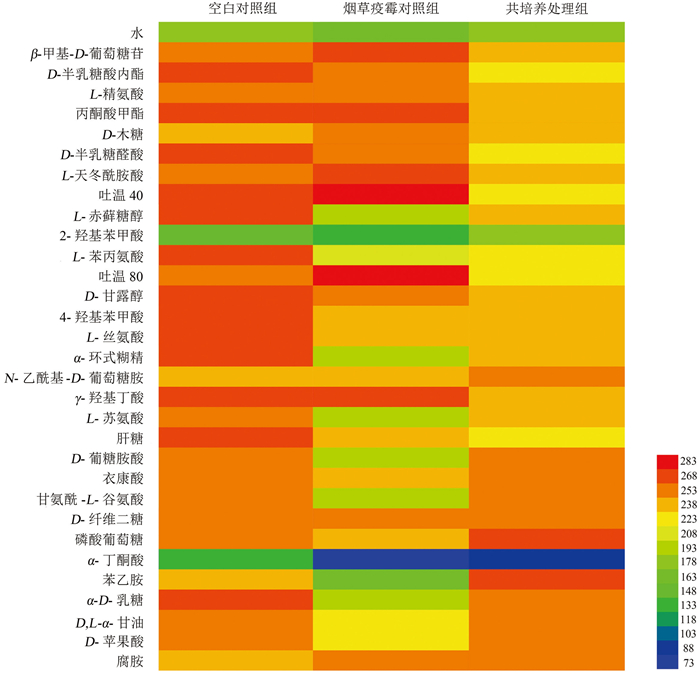
烟叶病际微生物在Biolog ECO微平板上代谢表型如图 4所示,31种碳源均能被各处理组的烟叶病际微生物代谢,其中普遍代谢程度较高的有β-甲基-D-葡萄糖苷、L-精氨酸、L-天冬酰胺酸、D-甘露醇、L-丝氨酸、N-乙酰基-D-葡萄糖胺、γ-羟基丁酸等14种碳源。
在空白对照组和烟草疫霉对照组中,空白对照组的叶际微生物对D-半乳糖酸内酯、L-赤藓糖醇、4-羟基苯甲酸、L-苯丙氨酸、L-丝氨酸、甘氨酰-L-谷氨酸、α-D-乳糖等24种碳源的利用率比烟草疫霉对照组高,二者对α-丁酮酸和2-羟基苯甲酸的利用程度均较低。
共培养处理组病际微生物对31种碳源的整体利用率低于空白对照组,其中氨基酸、羧酸类碳源受到的抑制最明显,仅对磷酸葡萄糖和苯乙胺的利用程度高于空白对照组。共培养处理组能高效代谢磷酸葡萄糖、甘氨酰-L-谷氨酸、α-D-乳糖、D-苹果酸、腐胺等13种碳源,代谢程度稍低的碳源有L-赤藓糖醇、L-苯丙氨酸、α-环式糊精、L-天冬酰胺酸等16种碳源,对2-羟基苯甲酸、α-丁酮酸的利用率最低。
2.1. 烟草疫霉菌丝生长速率和生物量
2.2. 烟草疫霉孢子囊数量及游动孢子释放情况
2.3. 致病性及病际微生物碳源代谢功能
-
烟草疫霉与茄科劳尔氏菌共培养时,菌丝生长速率加快,孢子囊数量与烟草疫霉对照组存在显著差异,可以正常产生休止孢,这一现象可能与病原菌争夺营养物质有关。共培养处理组病斑直径在发病后期与烟草疫霉对照组间不存在显著差异,但病斑四周黄化程度加重,病斑中心穿孔,病叶边缘反卷,表明共培养一定程度上增强了烟草疫霉的致病性。
Biolog ECO碳源代谢结果可以准确反映样本中微生物群落的代谢情况[21]。空白对照组对大多数碳源代谢水平高,如L-精氨酸、D-半乳糖酸内酯、L-赤藓糖醇、4-羟基苯甲酸等,其中糖类、氨基酸类容易被微生物所利用[22]。共培养处理组烟叶病际微生物对D-半乳糖醛酸、L-苯丙氨酸、α-丁酮酸[23]等多种碳源的代谢受到抑制,其中D-半乳糖醛是2-酮-3-脱氧-6-磷酸葡糖酸(KDPG)途径和糖醛酸途径中的关键中间产物,其代谢受抑制可能表明共培养条件下烟叶病际微生物的糖代谢途径存在异常。N-乙酰基-D-葡萄糖胺是细菌细胞壁的组分之一,共培养处理组对其代谢程度最高,表明该处理组烟叶的病际细菌群落增殖能力更好。
烟草疫霉能代谢PM1、PM2微平板上74%的供试碳源[24],包括琥珀酸、D-半乳糖、L-天冬氨酸、L-脯氨酸、D-海藻糖、D-山梨糖、D-葡萄糖醛酸、D-葡萄糖酸、乳酸等。在Biolog ECO微平板中,烟草疫霉对照组的烟叶病际微生物可高效代谢β-甲基-D-葡萄糖苷、L-精氨酸、丙酮酸甲酯、D-木糖、L-天冬酰胺酸、γ-羟基丁酸等部分碳源,这种代谢偏好可能受烟草叶际优势菌群影响,导致感烟草黑胫病烟叶在代谢不同碳源底物时与烟草疫霉偏好不同。与刘亭亭等[25]的研究结果相似,本研究中各处理组烟叶叶际微生物对多数氨基酸和糖类碳源代谢强度较高,仅对2-羟基苯甲酸、α-丁酮酸的代谢率低。此外,各处理组代谢水平较低的碳源,如2-羟基苯甲酸、L-赤藓糖醇、D-葡萄糖胺酸、α-丁酮酸与向立刚等[26]研究中感黑胫病烟株茎秆微生物不能代谢的碳源一致,各处理组对D-甘露醇、磷酸葡萄糖的代谢水平与其研究结果不同,这可能与烟秆和烟叶病际微生物群落中高丰度真细菌群落的组成差异有关。
烟草黑胫病在田间生产中发病较早,随后烟草青枯病快速爆发并与其混合发病,基于本文结果推测,土壤中残留的茄科劳尔氏菌会刺激烟草疫霉菌丝生长、产孢,并增强其致病性,一定程度上提前了病害爆发时间。本文关于烟草疫霉与茄科劳尔氏菌共培养后的烟叶病际微生物碳源代谢结果丰富了烟草疫霉碳代谢组学内容,文中对两病原菌共培养时生物学特性的研究结果为后续防控提供了理论基础。





 DownLoad:
DownLoad: