-
磷(P)是植物生长发育必需的营养元素之一,也是植物有机物的重要组成成分,广泛参与体内能量转移、信号转导、氧化还原等生理生化代谢过程.由于自然界土壤中能被植物直接吸收的无机磷酸盐(Pi)含量非常低,往往小于10 μmol/L[1],出现了土壤缺磷制约植物生长的现象,因此植物体演化出多种适应磷饥饿的机制,其中最重要的一种方式是通过菌根进行改善.世界上超过90%的陆生植物能够与土壤菌根真菌形成共生关系[2],由植物根表皮和菌根所富集的磷素通过相同或不同家族的多个磷转运蛋白基因进行吸收利用[3],其中起重要作用的是Pht1磷转运蛋白家族,它负责植物体磷的吸收和体内转运,属于MFS蛋白家族[4],可以利用渗透势梯度来运输溶质分子,且发现大部分Pht1磷转运蛋白基因的表达受低磷胁迫诱导[5-6].据国内外研究表明,已相继从模式植物和大量的农作物中克隆和鉴定了Pht1磷转运蛋白家族基因,如:拟南芥已分离到9个[7]、水稻有13个[8],番茄至少有5个[9]Pht1家族成员,由于木本植物基因组庞大和连锁图谱的不完整,使得对磷转运蛋白基因家族的克隆和功能研究等面临了一些困难.
马尾松(Pinus massoniana)是中国湿润亚热带地区分布最广、资源最多的针叶林类型,主要分布于南方省区,具有速生、丰产、适生性强、用途广等优良特性,是典型的外生菌根(Ectomycorrhizal fungi,ECM)树种[10].自然界生长的马尾松根末端不形成根毛结构,而与大量外生菌根真菌形成共生体系,其对水分、营养物质的吸收,绝大部分也是通过菌根结构来实现,因此外生菌根在马尾松耐低磷机制中起着重要作用.迄今为止,马尾松磷转运蛋白基因的克隆及表达分析尚属空白,特别是外生菌根对磷转运蛋白基因表达的影响未见报道.本研究利用耐低磷的优良马尾松家系,克隆、筛选出马尾松Pht1家族磷运转蛋白家族基因,并分析在不同磷水平、不同组织中菌根对其表达的影响,旨在为马尾松磷高效分子育种提供优良的候选基因,对解析马尾松耐低磷机制具有重要意义.
HTML
-
材料选自四川眉山市丹棱县杨场镇生长势优良的20年龄的马尾松种子,前期研究表明,该家系耐低磷能力强(待发表).在贵州大学温室中培育1年龄实生苗.供试土壤采自贵州贵阳市孟关林场,采集距土表层50 cm下的土壤作为育苗基质,pH值为5.08,有机质5.71 g/kg,全氮0.43 g/kg,碱解氮38.38 mg/kg,全磷0.12 g/kg,有效磷1.32 mg/kg,全钾4.23 g/kg,速效钾28 mg/kg,属低磷黄壤粘土,按体积9:1与河沙混合.基质、珍珠岩、育苗盘和培养钵等进行灭菌处理后待用.
试验菌种包括美味牛肝菌(Boletus edulis,北京农学院曹庆芹教授惠赠)和彩色豆马勃(Pisolithus tinctorius,贵州大学植物生理实验室),两种菌种按1:1混合施用.将基质进行拌菌处理,在距离土层表面2/3处埋入固体菌剂,距土表1/3处喷洒液体菌剂,再采用液体与固体菌剂混合接种1年生马尾松根系,移栽入土后覆上灭菌的珍珠岩.
试验设不接种对照组和外生真菌混合接种试验组.磷浓度条件分别为0,5,50,500,5 000 μmol/L,每组处理10株,重复4次,用1/2 Hoagland营养液浇灌.分别在第0,6,12,18,24,30 d采集不经磷处理的接种试验组和对照组根系,在第90 d收获不同磷水平处理后的根、茎、针叶组织.
-
用RNA试剂盒(Tiangen公司)配合RNA Reagent(Invitrogen公司)分别提取不同处理的马尾松根、茎、针叶的总RNA,用琼脂糖凝胶电泳以及紫外分光光度计检测其浓度和纯度,-80 ℃超低温冰箱保存.利用M-MLV反转录酶(Promega公司)合成cDNA第一链,置-20 ℃保存备用.
-
根据NCBI公布的植物Pht1磷转运蛋白家族的基因序列(包括菌根诱导型和菌根特异型),利用PrimerPlus5以及CODEHOP法大量设计简并引物进行克隆. PCR反应扩增程序:94 ℃预变性3 min;94 ℃变性30 s,54~61 ℃退火30 s,72 ℃延伸1 min,35个循环;72 ℃继续延伸7 min,4 ℃终止反应. PCR产物经1%琼脂糖凝胶电泳后回收、纯化,连接T载体(Promega公司),重组子转化到DH5α(Tiangen公司),在培养基(Ampicilin/X-gaL/IPTG)上筛选;挑取单个白色菌落PCR鉴定阳性克隆,抽提质粒,送生工生物工程(上海)公司测序.
-
利用BLAST程序推定、比对获得基因片段的氨基酸序列以及对Pht1编码蛋白功能域进行预测;通过Clustal W软件和GeneDoc 2.7.0将获得的基因与裸子、双子叶及单子叶代表植物的Pht1氨基酸序列进行多重比对分析;利用MEGA 5.0软件,选择NJ(Neighbor-Joining)法进行1 000次抽样,制作系统进化树.
-
以不同处理的马尾松根、茎、叶cDNA为模板,选用稳定表达的UBQ和18S基因作为内参,据获得的马尾松Pht1磷转运蛋白基因设计特异引物(表 1),对菌根化根系中不同时间的表达特征和不同磷水平的处理进行定量PCR检测,分析磷转运蛋白基因相对表达情况.半定量PCR分别调整cDNA模板浓度使内参基因表达量一致,PCR扩增后产物用1.5%的琼脂糖凝胶电泳检测.利用Gene Tools图像软件分析电泳条带的灰度值,每条差异基因的灰度值与相同处理内参基因条带灰度值之比代表基因的相对表达量.荧光定量PCR采用Power SYBR Green Master Mix试剂盒(ABI公司),反应在ABI 7500荧光定量PCR仪上进行.反应程序为:95 ℃变性3 min后,94 ℃ 20 s;56~58 ℃退火25 s;72 ℃延伸20 s共40次循环,绘制溶解曲线,重复3次,操作按程序说明进行.采用2(-ΔΔCt)法获得各基因相对内参基因的表达量,利用SPSS 13.0进行方差分析,Excel 2007作图.
1.1. 试验材料及处理
1.2. RNA提取及反转录
1.3. 磷转运蛋白基因的克隆
1.4. 生物信息学分析
1.5. 基因表达分析
-
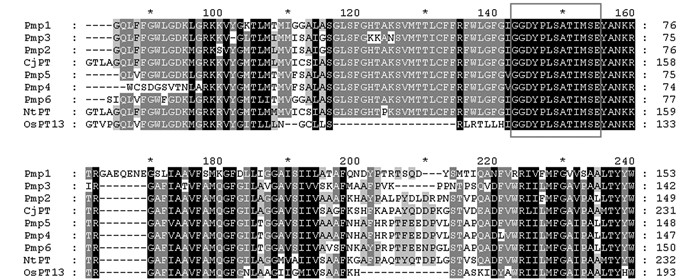
将获得的14条序列上传到NCBI数据库进行比对分析,结果表明,克隆的基因片段与日本柳杉(BAE92291)、烟草(AAF74025) 等植物的磷转运蛋白基因具有较高的相似性.将基因的氨基酸序列与CjPT(日本柳杉)、NtPT(烟草)、OsPT13(水稻)等植物的磷转运蛋白基因序列进行比对,发现其中有6个成员都具有Pht1家族磷转运蛋白基因的特征序列GGDYPLSATIMSE(图 1).将这6个基因片段分别命名为PmP1(826 bp)、PmP2(825 bp)、PmP3(829 bp)、PmP4(1 283 bp)、PmP5(1 284 bp)、PmP6(1 282 bp),成员间相似性达70%以上.此外,对编码蛋白功能域进行分析发现6个基因中均含有2A0109结构域,它是H2PO4-/H+转运子,属于MFS转运蛋白家族.
-
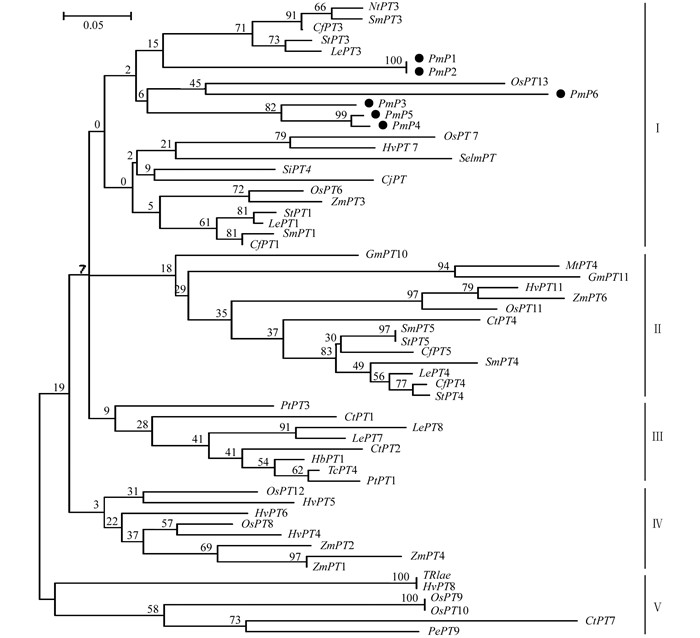
为探明马尾松与其他植物磷转运蛋白的进化关系,通过邻接法构建马尾松与53个Pht1磷转运蛋白基因的系统发育树(图 2).根据较近的共线性关系,各成员被划分为5个不同的亚组,马尾松6个Pht1磷转运蛋白基因的遗传距离较近聚为一个亚组,其中PmP1和PmP2彼此具有最高同源性,PmP3、PmP4和PmP5同源性较高,其亲缘关系较近的有番茄(Lycopersicon esculentum)中LePT3,马铃薯(Solanum tuberosum)中StPT3,辣椒(Capsicum frutescens)中CfPT3,茄子(Solanum melongena)中SmPT3和烟草(Nicotiana tabacum)中NtPT3,且均属于双子叶草本植物.而PmP6单独和单子叶植物水稻(Oryza sativa)中OsPT13聚为一类.获得的大部分马尾松Pht1家族磷转运蛋白与双子叶植物的亲缘关系要更近一些.
-
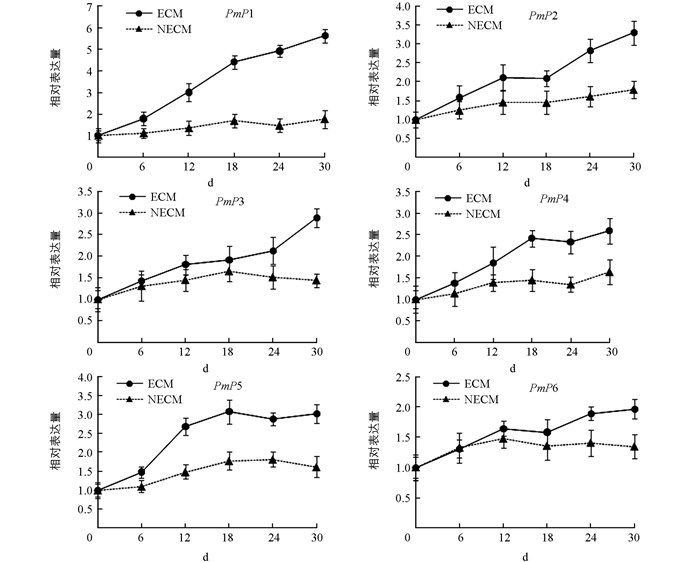
测定马尾松根部在不同处理时间(菌根接种0,6,12,18,24,30 d)的荧光定量相对表达量. 图 3表明,不同时间点PmP1~ PmP6在马尾松根部的表达量不同,随时间延续所有基因的表达量都呈上升趋势.根中PmP1~PmP6的表达均受外生菌根的诱导,其表达量都显著高于不接种对照,其中PmP1在第30 d的表达量比无侵染对照显著性提高了5倍左右,而PmP2~PmP6表达量呈曲线式上升,比不接种对照增强了约2~3倍.
-
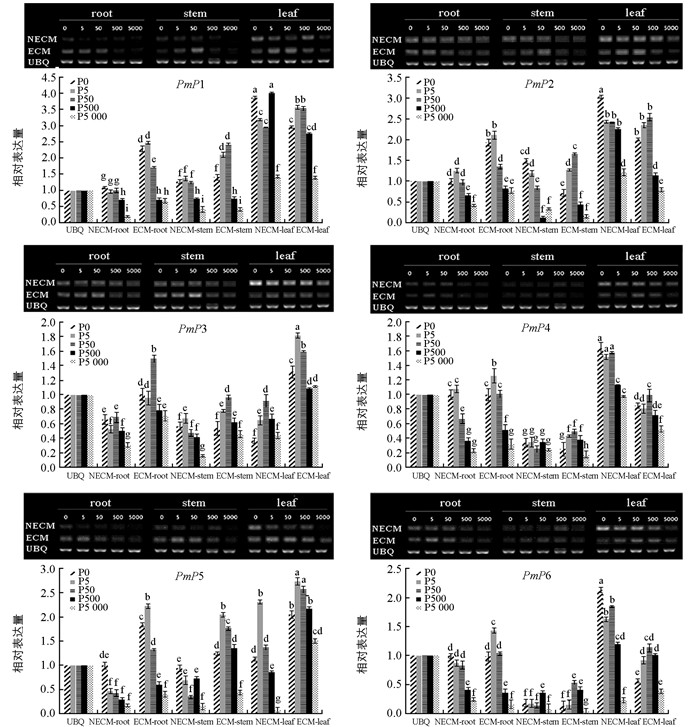
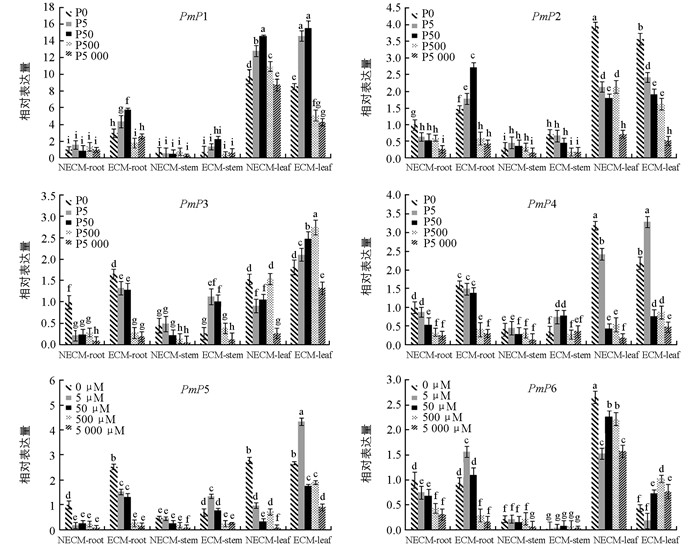
利用半定量PCR检测马尾松Pht1家族基因在不同组织、不同磷水平下的相对表达情况.结果显示(图 4),6个基因在根、茎、针叶中都有表达,但不同处理下相同部位表达量不同;相同处理后不同组织其表达量也不同. PmP1~PmP6在未接种菌剂的马尾松针叶中高量表达,其次为根,茎中最少;接种菌根菌后,根和茎中的表达量比未接种处理的均有不同程度的上调,针叶中PmP4和PmP6表达量呈显著下调趋势.磷浓度变化影响了马尾松Pht1磷转运蛋白基因表达,包括根、茎、针叶中的PmP1~PmP6在0~50 μmol/L低磷浓度范围内表达量显著高于500~5 000 μmol/L高磷浓度范围的表达,特别是接种菌根真菌比不接种的根系对低磷水平的响应更为明显.在极度缺磷(0 μmol/L)的条件下,未接种菌根菌的针叶中PmP1、PmP2、PmP4、PmP6表达量均显著性高于接种处理.
-
实时荧光定量PCR检测马尾松磷转运蛋白基因相对表达量(图 5)的结果表明,未接种菌根菌的针叶中PmP1表达量显著高于根和茎,接种后在0~50 μmol/L低磷浓度范围内根系的相对表达量是未处理的3~6倍,茎、叶中变化幅度不大,PmP2表达模式与PmP1相似.在0~50 μmol/L磷浓度范围内,接种菌剂植株中PmP3表达量高于未接种处理,PmP4和PmP5在根、茎中表达模式与此类似,其在5 μmol/L磷浓度处理的菌根化针叶中表达量提升最显著.与未接种对比,PmP6在接种处理后针叶的表达量有明显下降,特别是在0~5 μmol/L磷浓度范围内,其相对表达量比未接种的下降了5~7倍.极度缺磷(0 μmol/L)状况下,PmP2、PmP4、PmP6在未接种的针叶中表达量显著性最高,接种后下调表达.综上所述,荧光定量表达分析的结果与半定量表达分析比较一致,外生菌根能诱导PmP1~PmP6在马尾松根、茎、针叶中表达量的变化,在磷浓度0~5 μmol/L间,大部分Pht1磷转运蛋白基因表达量明显增强,而磷浓度在500~5 000 μmol/L范围内下调表达.
2.1. 马尾松磷转运蛋白基因片段的克隆
2.2. 系统进化分析
2.3. 菌根化根系中PmP1~ PmP6在不同时间的表达特征
2.4. 半定量表达分析
2.5. 荧光定量表达分析
-
磷转运蛋白基因是植物与菌根真菌共生过程中所特异驱动的基因,它编码植物菌根吸收途径中磷素利用的关键蛋白[11-12],其中Pht1家族是当今菌根互作研究中最重要的磷转运蛋白[9, 13].本研究利用简并引物分离克隆了菌根化马尾松Pht1磷转运蛋白家族基因,筛选出Pht1家族的6个基因片段,其氨基酸序列之间有较高同源性,均具有GGDYPLSATIMSE的保守序列. Karandashov等[11]研究表明,所有Phtl磷转运蛋白基因都具有GGDYPLSxxIxSE的特征序列,证明了PmP1~PmP6属于Pht1家族成员.分子系统分析表明,PmP1~PmP6基因属于一个亚组,它们与StPT3(马铃薯)、LePT3(番茄)、CfPT3(辣椒)、SmPT3(茄子)及NtPT3(烟草)等茄科双子叶植物亲缘关系最近,其中马铃薯StPT3是第一个被克隆了的在植物与真菌间起作用的Pht1磷转运蛋白基因,主要在包含丛枝袍囊的根皮层细胞中表达,该基因被证实属于高亲和力转运蛋白[14];后来,LePT3、CfPT3、SmPT3和NtPT3基因也都被证实可以被菌根诱导表达[9].而PmP6单独与OsPT13(水稻)聚为一类,后者也被证实属于菌根诱导型.因此,从接种菌根菌的马尾松根系中获得的PmP1~PmP6极有可能是能被菌根诱导的磷转运蛋白基因.
根据植物的进化演变,裸子植物比被子植物要古老,对磷素的吸收和转运起作用的马尾松Pht1家族磷转运蛋白却和被子植物中双子叶类的亲缘关系很相近.即使是进化树上相距很远的基因OspT13和TRIae,其氨基酸序列相似性也达到55.65%,这反映出植物菌根磷酸盐转运蛋白在进化上具有较高的保守性.说明马尾松Pht1家族的磷转运蛋白基因在漫长的进化过程中极其保守.有分析还发现StPT3启动子区有一个类转座子序列元件,表明StPT3是由普通Pht1转运蛋白在启动区突变得到的[14],而在进化树中与其相邻的另一个亚组中的StPT5、LePT4、OsPT11和MtPT4等则是从同一古老基因进化而来[9] [15-16],对马尾松Pht1磷转运蛋白演化的研究有新的启示.
基因表达分析证明,无论是否受外生真菌侵染,PmP1~PmP6在根、茎、叶中都有表达,接种后PmP1~PmP6在根部表达量有不同程度上调,这与Nagy等人的结果一致[9],但茎和针叶中的表达量也有变化,与其他研究结果不同.有报道认为,绝大部分克隆了的Pht1家族磷转运蛋白基因主要是在根部起作用,负责磷的吸收和转运[3],如:拟南芥有8个基因[7],水稻中有10个基因都在根部表达[8].其在茎和针叶中的表达和变化可能与磷在植物体内的分布有关,各基因在苗期与成熟期中组织间表达量的不同,可能是由生长过程中磷吸收量所致[17].缺磷环境中,与未接种菌根菌处理相比,接种根系中PmP1~PmP6上调表达,而针叶中表达量下降,这种低磷胁迫下转录水平的变化可能由根系共生的菌根和土壤中可溶性无机磷的浓度综合调控.菌根真菌激活了低磷环境中马尾松根部磷转运蛋白基因的显著表达,茎和针叶中其表达量也发生改变,可能是Pht1家族基因的组织特异性和时空特异性的表达,提示PmP1~PmP6在马尾松吸收、转运磷的过程中可能具有不同作用.马尾松Pht1家族成员PmP1~PmP6在0~5 μmol/L低磷浓度范围内有不同程度的上调表达趋势,而在500~5 000 μmol/L高磷浓度范围中下调表达,与Nagy研究结果一致,菌根磷转运蛋白基因的表达与磷水平相关,与磷饥饿途径无关[12].
本研究克隆、筛选了6个马尾松Pht1家族磷转运蛋白基因,PmP1~PmP6受外生菌根的诱导,其在不同磷浓度处理下根、茎、针叶中表达量不同,具体表现为低磷水平下被高效激活,高磷水平下表达反被抑制.这为进一步研究菌根诱导型Pht1磷转运蛋白家族基因全长和功能奠定了基础,也为全面解析马尾松耐低磷机制提供了新信息.






 DownLoad:
DownLoad: