-
草地贪夜蛾(Spodoptera frugiperda)是一种全球性迁飞的鳞翅目农业害虫,主要采食玉米、水稻、高粱、甘蔗、花生和大豆等多种农作物,危害巨大.该害虫自2019年1月入侵中国以来,已经蔓延至全国25个省份,对我国粮食生产造成了重大的威胁.
本课题组前期对入侵重庆地区的草地贪夜蛾肠道优势微生物进行了初步的分离鉴定,并对取食不同食物(高粱、玉米)的草地贪夜蛾肠道微生物群也进行了比较,对研究草地贪夜蛾核心肠道微生物提供了重要的数据支持[1-5].但前期实验对象均为幼虫样本,且都来自于重庆地区,采样的地域范围相对较小且样本的发育阶段单一,因此对全面认识草地贪夜蛾肠道微生物的组成有一定的局限性.有研究表明,肠道微生物与昆虫的生长、生殖系统及消化系统发育、运动能力、迁徙聚集等行为有着密切的关系[6-8].云南是草地贪夜蛾侵入我国的第一个受灾省份,部分地区全年气候温暖,是草地贪夜蛾的理想生存地区,因此云南部分地区可能成为草地贪夜蛾长期定殖和越冬的地区.所以充分了解入侵云南地区的草地贪夜蛾肠道微生物的组成以及丰度,有助于后续针对于草地贪夜蛾的生物学适应性的研究.
本研究在课题组前期研究的基础上,通过传统培养法结合16S rDNA测序对云南蒙自地区玉米地草地贪夜蛾幼虫和成虫的肠道优势菌进行了初步分离鉴定,并对不同地区草地贪夜蛾肠道优势细菌类型进行了比较,以期为草地贪夜蛾肠道微生物的后续研究奠定基础.
HTML
-
云南省蒙自县草坝镇玉米地的草地贪夜蛾成虫及幼虫.
-
牛肉膏蛋白胨培养基(NA):牛肉膏5.0 g,蛋白胨10.0 g,氯化钠5.0 g,琼脂20.0 g,蒸馏水定容至1 000 mL;
2×YT生长培养基:胰蛋白胨16.0 g,酵母提取物10.0 g,氯化钠5.0 g,琼脂20.0 g,蒸馏水定容至1 000 mL;
脑心浸液培养基(BHI):蛋白胨10.0 g,脑心浸粉12.5 g,磷酸氢二钠2.5 g,氯化钠5.0 g,葡萄糖2.0 g,琼脂20.0 g,蒸馏水定容至1 000 mL;
改良的LB培养基(LBG):胰蛋白胨10.0 g,酵母提取物5.0 g,氯化钠5.0 g,琼脂20.0 g,蒸馏水定容至1 000 mL.
-
SDS,CTAB,NaCl,Tris,EDTA,蛋白酶K、蔗糖和溶菌酶均购自生工生物工程(上海)股份有限公司;苯酚、氯仿和异戊醇均购自重庆川东化工(集团)有限公司;PCR扩增引物27F:5'-AGAGTTTGAT CCTGGCTCAG-3';1492R:5'-GGTTACCTTGTTACGACTT-3',由生工生物工程(上海)股份有限公司合成.
-
采样自云南省蒙自县.将幼虫置于超净工作台中,用75%乙醇对幼虫进行表面消毒后,剖取其中肠置于装有0.7 mL灭菌磷酸缓冲液(PBS)的1.5 mL离心管中,充分震荡混合制成肠道内容物悬液.
-
取50 μL肠道内容物悬液加入450 μL磷酸缓冲液(PBS),梯度稀释后得到10-1~10-9倍稀释的肠道内容物悬液,将10-1~10-9的稀释液各取100 μL在NA,2×YT,LBG以及BHI固体培养基上涂板,30 ℃培养箱培养24~72 h后挑取不同形态特征的单菌落划线培养,通过多次单菌落划线以获得纯分离株.
-
细菌的分子鉴定主要参考前期课题组发表的文章[5]中的方法,得到的PCR产物送至生工生物工程(上海)股份有限公司测序.
-
将测序结果在NCBI(https://www.ncbi.nlm.nih.gov)中进行序列比对,下载同源性最高的序列,使用MEGA 5.0进行多重序列比对,并构建系统发育树进行分析.同时在RDP数据库中获得的16S rDNA基因序列用Classifer程序(https://rdp.cme.msu.edu/Classifer/Classifer.jsp)进行比对,获得同源性较高序列的相关信息.
1.1. 材料
1.2. 实验用的培养基
1.3. 主要试剂
1.4. 草地贪夜蛾幼虫和成虫中肠内容物的收集
1.5. 肠道细菌的分离培养
1.6. 细菌的分子鉴定
1.7. 数据分析
-
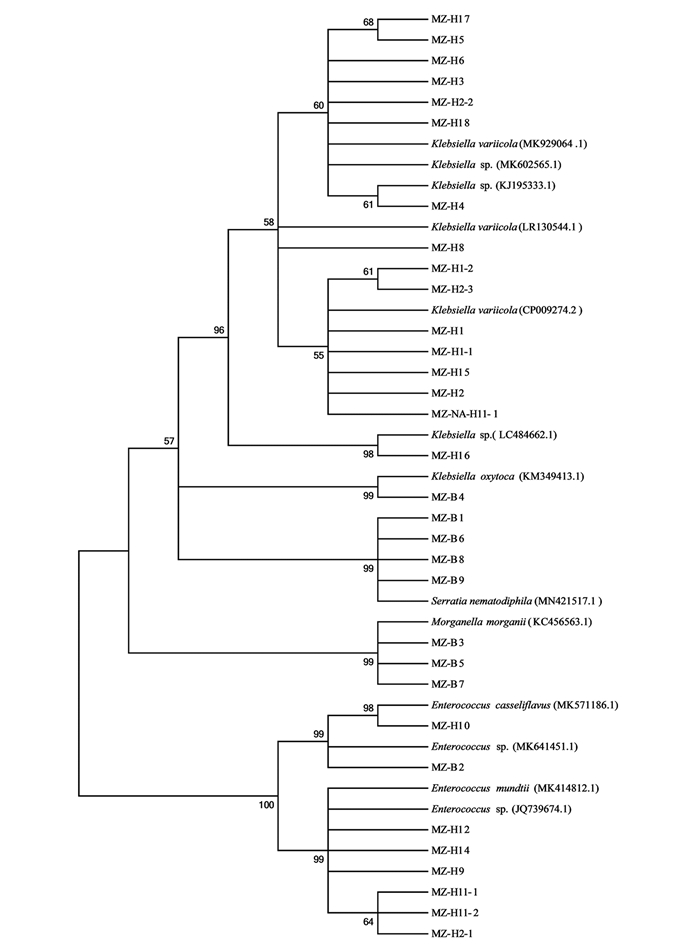
采用NA,2×YT,LBG以及BHI培养基,从云南蒙自地区的玉米地采集的草地贪夜蛾幼虫肠道中分离获得32个分离株.通过细菌通用引物27F/1492R对32个分离株进行PCR扩增测序获得其16S rDNA序列.将所有测序结果利用Blastclustx进行同源性聚类分析,同源性大于97%的聚为同一操作分类单元(operational taxonomic units,OTU),共获得4个聚类单元OTU-L1-L4(表 1).
将测序结果分别在GenBank和RDP两个数据库比对获得相关信息.比对结果显示,本次从云南蒙自地区的草地贪夜蛾幼虫肠道分离的32个分离株中,MZ-H1,MZ-H1-1,MZ-H1-2,MZ-H2,MZ-H2-2,MZ-H2-3,MZ-H3,MZ-H4,MZ-H5,MZ-H6,MZ-H8,MZ- NA-H11-1,MZ-H15,MZ-H16,MZ-H17,MZ-H18和MZ-B4共17个分离株为克雷伯氏菌属(Klebsiella);MZ-B1,MZ-B6,MZ-B8和MZ-B9共4个分离株为沙雷氏菌属(Serratia);MZ-H2-1,MZ-H9,MZ-H10,MZ-2XYT- H11-1,MZ-H11-2,MZ-H12,MZ-H14和MZ-B2共8个分离株为肠球菌属(Enterococcus);MZ-B3,MZ-B5和MZ-B7共3个分离株为摩根菌属(Morganella).在所有分离株中,克雷伯氏菌属(Klebsiella)的丰度最高,为53.1%,沙雷氏菌属(Serratia)和摩根菌属(Morganella)是前期实验未在其他地区草地贪夜蛾中发现的新分离株(表 1).
为进一步了解云南地区草地贪夜蛾肠道优势菌的遗传多样性,对32个肠道细菌分离株进行了系统发育分析.对分离株16S rDNA序列进行BLAST比对分析,采用MEGA 5.0软件邻接法构建系统发育树(图 1).从图 1可以看出,同一OTU的细菌分离株在同源进化关系上存在一定的差异,即种间多样性较大.例如,在分离到的17个克雷伯氏菌属分离株中,MZ-B4和MZ-H16与其他15个分离株归为一个聚类单元,但是存在较远的亲缘关系.
-
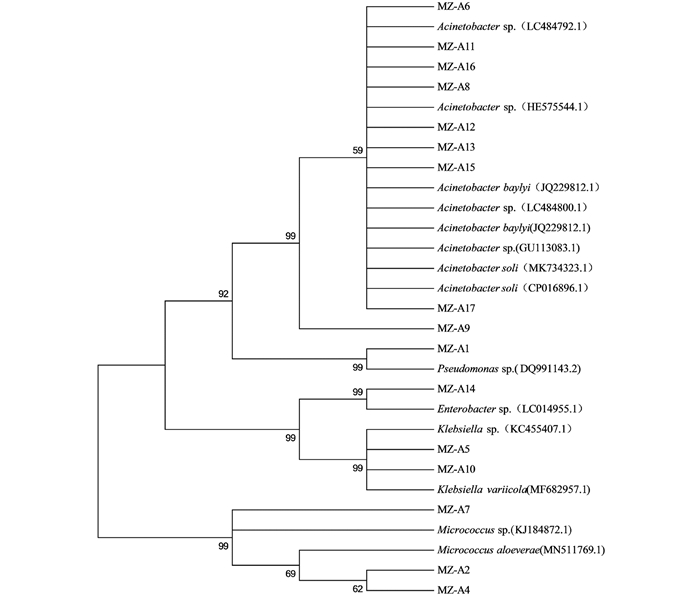
采用多种培养基,从云南蒙自地区采集的草地贪夜蛾成虫肠道中获得16个细菌分离株.通过细菌通用引物27F/1492R对16个分离株进行PCR扩增测序获得其16S rDNA序列.将所有测序结果利用Blastclustx进行同源性聚类分析,同源性大于97%的聚为同一OTU,共获得5个聚类单元OTU-A1-A5(表 2),并将测序结果分别在GenBank和RDP两个数据库比对获得相关信息.
比对结果显示,本次从云南蒙自地区的草地贪夜蛾成虫肠道分离的16个分离株中,MZ-A6,MZ-A8,MZ-A9,MZ-A11,MZ-A12,MZ-A13,MZ-A15,MZ-A16以及MZ-A17共9个分离株聚类到OUT-A1,为不动杆菌属(Acinetobacter);MZ-A2,MZ-A4和MZ-A7共3个分离株聚类到OUT-A2,为微球菌属(Micrococcus);MZ-A5以及MZ-A10两个分离株聚类到OUT-A3,为克雷伯氏菌属(Klebsiella);MZ-A14为肠杆菌属(Enterobacter),MZ-A1为假单胞菌属(Pseudomonas),其中不动杆菌属(Acinetobacter)的丰度最高,为56.0%.为进一步了解云南地区草地贪夜蛾成虫肠道优势菌的遗传多样性,将16个肠道微细菌分离株进行了系统发育分析.对分离株16S rDNA序列进行BLAST比对分析,采用MEGA 5.0软件邻接法构建系统发育树,结果见图 2;与同源性聚类分析的结果一致,分离到的16个菌株在系统发育树上分为5个属,分别为不动杆菌属(Acinetobacter)、克雷伯氏菌属(Klebsiella)、微球菌属(Micrococcus)、假单胞菌属(Pseudomonas)和肠杆菌属(Enterobacter);其中不动杆菌属的分离株MZ-A9与其他不动杆菌属的分离株存在一定的遗传距离.
2.1. 草地贪夜蛾幼虫肠道细菌分离鉴定
2.2. 草地贪夜蛾成虫肠道细菌分离鉴定
-
本实验通过纯培养的方法,分离培养并初步鉴定了云南蒙自地区玉米地中草地贪夜蛾幼虫及成虫的肠道细菌.在幼虫肠道中共分离鉴定到了4个属的细菌,分别为克雷伯氏菌属(Klebsiella)、肠球菌属(Enterococcus)、沙雷氏菌属(Serratia)和摩根菌属(Morganella),其中克雷伯氏菌属(Klebsiella)的丰度最高,为53.1%.值得关注的是本课题组前期在重庆各地区的草地贪夜蛾幼虫样本中,都分离鉴定到了克雷伯氏菌属(Klebsiella)细菌且丰度较高. Jones等[9]学者从美国地区草地贪夜蛾幼虫也分离到了克雷伯氏菌属(Klebsiella),Acevedo等[10]学者证明分离于草地贪夜蛾的克雷伯氏菌属(Klebsiella)细菌分离株能够显著抑制植物过氧化物酶并上调植物的胰蛋白酶抑制剂的表达,从而降低植物的防御反应提高草地贪夜蛾的存活率.推测草地贪夜蛾幼虫的肠道微环境适于克雷伯氏菌属(Klebsiella)的定殖,克雷伯氏菌属(Klebsiella)细菌通过抑制植物的防御反应促进草地贪夜蛾幼虫适应环境,从而达到互利的结果.本课题组的研究结果显示在云南与重庆地区玉米地的草地贪夜蛾幼虫肠道中都是以克雷伯氏菌属(Klebsiella)为主要优势菌.但是在云南草地贪夜蛾幼虫肠道中分离到的沙雷氏菌属(Serratia)和摩根菌属(Morganella),在重庆草地贪夜蛾幼虫肠道中未分离到,而在重庆草地贪夜蛾幼虫肠道中分离到的不动杆菌属(Acinetobacter)、假单胞菌属(Pseudomonas)和肠杆菌属(Enterobacter)等细菌的丰度都较高,但是在云南草地贪夜蛾幼虫肠道中未分离到.这表明云南和重庆地区的草地贪夜蛾幼虫肠道中的主要优势菌相似,但是优势菌种类以及丰度存在一定的差异.
在草地贪夜蛾成虫肠道中共鉴定到了5个属的细菌,分别为不动杆菌属(Acinetobacter)、克雷伯氏菌属(Klebsiella)、微球菌属(Micrococcus)、假单胞菌属(Pseudomonas)和肠杆菌属(Enterobacter),其中丰度最高的为不动杆菌属(Acinetobacter),丰度为56.0%.有研究报道昆虫肠道微生物会因昆虫的发育阶段不同而出现不同组成结构[11-13].此前暂未见对入侵中国的草地贪夜蛾成虫肠道细菌分离鉴定相关研究的报道,因此草地贪夜蛾成虫与幼虫肠道微生物之间的相关性暂不明确.在本研究结果中成虫肠道中不动杆菌属(Acinetobacter)的丰度最高,而在幼虫肠道中丰度最高的是克雷伯氏菌属(Klebsiella);在云南的草地贪夜蛾幼虫与成虫肠道中都分离出克雷伯氏菌属(Klebsiella),但在成虫中暂未鉴定到不动杆菌属(Acinetobacter)、肠球菌属(Enterococcus)和摩根菌属(Morganella).这些结果表明草地贪夜蛾幼虫与成虫肠道中主要的优势菌存在差异. Takashima[14]的研究证明果蝇的肠道在蛹期时会发生巨大变化,其肠道微生物也会随之改变,因此推测草地贪夜蛾的肠道微环境在幼虫发育至成虫过程中发生了变化,从而影响了肠道微生物的组成结构. Schretter等[15]学者的研究表明,果蝇成虫肠道中的短乳杆菌(Lactobacillus brevis)表达的木糖异构酶可以降低果蝇海藻糖水平,从而降低生成神经递质章鱼胺的神经元活性,进而降低果蝇成虫运动过度活化的行为.草地贪夜蛾成虫与其肠道细菌是否存在相似作用的关系,待后续进一步探究.
草地贪夜蛾作为一种食性较广的昆虫,具有很强的迁飞和繁殖能力,对农业生产存在极大的危害,所以草地贪夜蛾是我国未来害虫防治的重点.草地贪夜蛾对有机磷类、有机氯类和拟除虫菊酯类等传统农药具有一定的抗性[16],这必定会导致杀虫剂的持续使用,而对环境造成巨大的影响,这进一步增大了对草地贪夜蛾的防控难度.据Almeida等[17-21]学者报道,在抗药性较强的害虫肠道中分离出的某些细菌分离株对农药有降解作用,并且可以影响农药在体内的代谢,其中主要是假单胞杆菌属(Pseudomonas)以及肠杆菌属(Enterobacter)的细菌对某些农药具有抗性. Muhammad等[22]学者的研究表明,肠道微生物群会通过调节昆虫的免疫能力来提高其生存能力. Thakur等[23]学者在斜纹夜蛾中分离到的阴沟肠杆菌(Enterobacter cloacae)能显著影响其生长发育和免疫调节,并且对斜纹夜蛾有较大的致死能力.本课题组前期在草地贪夜蛾的幼虫肠道中鉴定到了肠杆菌属(Enterobacter)以及假单胞菌属(Pseudomonas)等细菌,草地贪夜蛾的抗药性与其肠道微生物之间是否存在关系,后续也需要进行进一步的探究.






 DownLoad:
DownLoad: