-
开放科学(资源服务)标识码(OSID):

-
应激是机体在不良条件下做出的生理反应,根据应激持续时间长短可分为慢性应激和急性应激. 慢性应激是机体长时间受到不良因素刺激,打破机体原有稳态平衡后出现的一系列异常反应. 在畜禽养殖中慢性应激诱发机体功能紊乱,导致机体对营养物质的吸收和消化能力下降、饲料转化率降低和生长缓慢,从而造成动物经济效益降低,给养殖业带来不利影响.
机体营养物质吸收主要由小肠负责,食物在小肠的加工处理下大部分营养物质被吸收进入血液,运输至身体各部位,而大肠负责吸收剩余的水分和无机盐等微量物质[1]. 在生理条件下,肠屏障选择性参与保护身体免受外部环境病原体和有害成分渗透之间的正确平衡. 营养物质的选择性吸收是通过细胞间或跨细胞运输实现的,而有害物质和废物则通过粪便从胃肠道排出[2]. 肠道健康包括完整的绒毛结构和肠上皮屏障,发育良好的肠绒毛组织结构促进营养吸收[3]. 肠屏障的完整性部分是由一组完整的细胞蛋白、紧密连接复合物、细胞骨架微管和细丝赋予的,它们维持上皮细胞之间牢固的细胞连接,提供机械屏障保护机体,选择性通过对机体有利的物质[4-5]. 前人研究发现,慢性应激会破坏机体肠黏膜屏障,使肠黏膜屏障上皮细胞间紧密连接蛋白表达水平下降、黏膜上皮间杯状细胞数量减少、基底层细胞增殖变慢等[6]. Siddiqui等[7]研究发现在慢性应激下禽类肠道会发生损伤,小肠绒毛受损脱落、通透性增加. 与此同时,相关研究表明慢性应激也会致使机体炎症水平上升[8]. 在鸡慢性热应激和盲肠菌群结构研究中发现,养殖过程中产生的高密度、热应激、外伤、传染病及菌群结构变化等不可预知慢性条件长期刺激下,对畜禽健康及经济效益都有严重影响[9-11]. 已有研究表明,慢性不可预测应激导致肉鸡屏障功能破坏,表现为通透性增加、促炎细胞因子mRNA水平升高、紧密连接蛋白丰度降低和黏膜分泌型免疫球蛋白A(Secretory immunoglobulin A,sIgA)减少[12].
屏障受损会导致肠道渗漏,使管腔内的微生物产物和毒素侵入固有层甚至体循环,并激活炎症免疫反应,引起炎症性肠病(Inflammatory Bowel Disease,IBD)[13]. 肠黏膜是免疫的第一道屏障,防御病原菌在肠道黏附和定植[14]. 因此,保持肠道屏障完整性和维护肠屏障功能正常,对畜禽健康意义重大. 由于慢性应激致肠道屏障损伤机理仍不清楚,因此研究慢性应力损伤机理,寻找有效缓解措施尤为重要.
本研究以小鼠为研究对象,参考廖莎等[15]的实验构建慢性应激模型,通过组织化学染色、免疫组化和酶联免疫吸附技术(Enzyme-linked Immunosorbent Assay,ELISA)等技术,研究慢性应激对小鼠生长发育、激素水平、屏障损伤和自我更新等过程的影响,揭示畜禽养殖过程中慢性应激对机体产生的有害作用,阐明慢性应激对小肠屏障完整性破坏的机制,为养殖人员在养殖业中减少慢性应激,提高经济效益,实现健康养殖提供理论依据.
HTML
-
去甲肾上腺素(NE)、皮质醇ELISA检测试剂盒购于武汉云克隆生物有限公司(货号:CEA907Ge、(CEA540Ge);MDA,T-AOC,CAT,SOD,PAS染色液购于北京索莱宝生物有限公司(货号:BC0025,BC1315,BC0205,BC0175,G1281);GSH-Px购于南京建成生物有限公司(货号:A001-3-2);苏木素-伊红染色液(HE)购于白鲨生物有限公司(货号:BL700B);兔抗PCNA购于沈阳万类生物有限公司(货号:WL03213);兔抗ZO-1购于武汉三鹰生物技术有限公司(货号:21773-1-AP);TUNEL免疫荧光试剂盒购于赛维尔生物有限公司(货号:G1502-50T);UltraSensitiveTM SP(鼠/兔)IHC Kit试剂盒购于福州迈新生物科技有限公司(货号:KIT-9710);光学显微镜购自日本Olympus公司;石蜡包埋机、切片机2235、组织摊片机购于德国莱卡公司;艾本德高速低温离心机购于德国Eppendorf公司.
-
8周龄雄性昆明鼠24只,体质量(30±2) g,饲养于温度(20±2) ℃、湿度(50±5) %的实验动物饲养房中. 适应1周后将其分为2组,对照组不做任何处理,实验组每天9:00-14:00束缚于小鼠自制固定器中5 h,期间两组小鼠均禁食禁水,持续24 d. 实验过程中记录体质量、食物摄入量变化.
-
实验第24 d,将对照组、实验组小鼠在完成慢性束缚应激后进行旷场试验(Open Field Test,OFT). 使用SMART 3.0(SMART 3.0,西班牙)系统记录小鼠在2 min内的探索行为,包括活动总距离、中央区域活动距离、边缘区域活动距离.
-
试验结束后取小鼠血液,经4 ℃、3 000 r/min离心10 min收集血浆保存于-80 ℃冰箱中. 用于NE,CORT,IL-18,TNF-α,IL-1β,IL-10检测,在酶标仪450 nm波长处测量各孔吸光度值,按照说明书计算最终浓度.
-
取小肠组织用4%多聚甲醛固定液固定,制作成石蜡切片,烘干、脱蜡、并进行浓度梯度酒精复水,经HE染色后封片于显微镜下镜检组织学变化. 使用Image-pro plus 6.0软件统计每张图片中肠绒毛高度和肠隐窝深度,再计算V/C比值.
-
取血浆离心后根据MDA,T-AOC,GSH-Px,CAT,SOD试剂盒说明书进行检测.
-
小肠组织经石蜡包埋制作成切片后,用1×柠檬酸钠抗原修复液加热20 min后自然冷却至室温,PBS洗涤3次后封闭非特异性抗原,一抗兔源PCNA∶PBS体积稀释比(1∶150)、兔源ZO-1∶PBS体积稀释比(1∶400),4 ℃ 12 h孵育一抗;二抗山羊抗兔IgG∶PBS体积稀释比(1∶200),37 ℃孵育1 h,二氨基联苯胺(3,3-diaminobenzidine,DAB)显色后进行苏木素染液复染3 min,按浓度梯度酒精脱水后中性树胶封片观察,显微镜下拍照记录. 采用Image-Pro Plus 6.0软件计算组织中PCNA、ZO-1阳性区域平均吸光度值. PAS染色使用索莱宝试剂盒,切片常规脱蜡复水,经碘酸氧化后雪夫试剂染色,苏木素染液复染,1%盐酸酒精分色,浓度梯度酒精脱水,二甲苯透明,中性树胶封片后拍照记录.
-
将切片进行抗原修复后通透平衡,使用标记工作液进行标记反应1 h,PBS洗涤3次,每次5 min. 采用DAPI细胞核染色8 min,抗荧光淬灭封片剂封片,立即在荧光显微镜下观察拍照. 采用Image-Pro Plus 6.0软件计算组织中红色荧光阳性区域平均吸光度值.
-
实验数据显著性差异分析参考统计软件SPSS 27.0的独立样本T值检验(T-TEST),在所有分析中ns表示p>0.05数据间差异不具有统计学意义;*表示p<0.05数据间差异具有统计学意义,**表示p<0.01数据间差异具有统计学意义,***表示p<0.01数据间差异具有统计学意义.
1.1. 主要试剂与仪器
1.2. 方法
1.2.1. 实验动物与实验设计
1.2.2. 旷场实验
1.2.3. ELISA检测
1.2.4. 小鼠小肠HE染色
1.2.5. 小鼠血浆氧化应激指标检测
1.2.6. 小鼠小肠免疫组化和PAS染色
1.2.7. 小鼠小肠TUNEL免疫荧光
1.2.8. 数据统计与分析
-
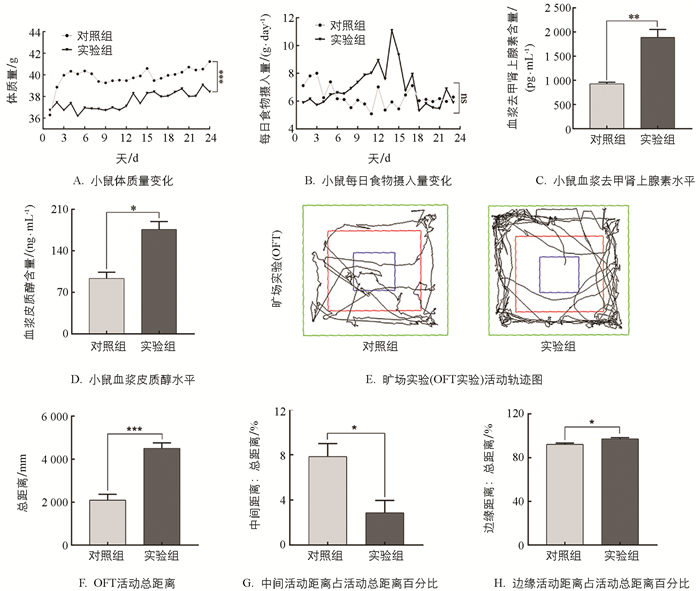
与对照组相比,实验组小鼠体质量极显著下降5.59%(p<0.001),试验期间两组采食量差异不具有统计学意义(p>0.05,图 1A、1B). 实验结束后检测两组血浆中去甲肾上腺素(Norepinephrine,NE)和皮质醇(Cortisol,CORT),验证机体是否处于应激状态. 实验发现,实验组较对照组NE含量显著上升102.95%(p<0.01)、CORT含量显著上升95.29%(p<0.05),提示慢性束缚应激模型构建有效(图 1C、1D). 旷场实验结果发现,实验组小鼠较对照组活动总距离显著增加135.25%(p<0.001),而中心区域活动距离减少63.5%(p<0.05);实验组边缘区域活动距离增多5.41%(p<0.05),表明实验组小鼠有明显焦虑样行为(图 1E、1F、1G、1H).
-
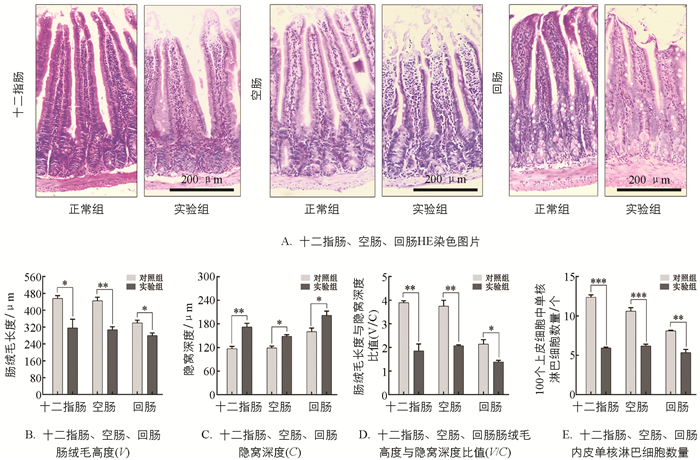
小肠组织经HE染色显示组织结构无明显病变(图 2A). 对肠绒毛和隐窝进行测量(图 2B、2C、2D),结果显示与正常组相比实验组各肠段肠绒毛高度(Villi,V)分别减少30.96%、31.12%、17.73%,差异具有统计学意义(p<0.05);隐窝深度(Crypt,C)分别增加46.90%,27.74%,26.01%(p<0.05);肠绒毛高度和隐窝深度比值(V/C)降低43.96%(p<0.05). 随后进行上皮单核细胞统计,在图 2E中发现实验组上皮单核细胞数量低于对照组51.95%,41.67%,34.07%(p<0.01),提示慢性束缚应激造成小鼠肠道机械屏障损伤.
-
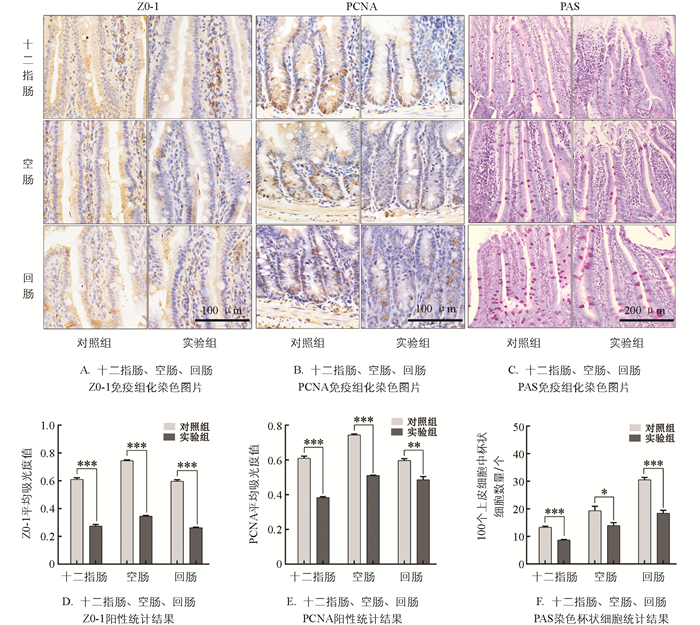
十二指肠、空肠、回肠免疫组化实验结果显示,实验组小肠组织紧密连接蛋白ZO-1表达显著低于对照组55.00%,44.89%,56.19%(p<0.001,图 3A、3D);细胞核增殖抗原(PCNA)阳性表达细胞极显著低于对照组37.13%,29.74 %,18.45%(p<0.01,图 3B、3E)). 通过PAS染色检测杯状细胞数量,结果显示与对照组相比,实验组杯状细胞数量显著减少34.74%,27.78%,39.47%(p<0.05,图 3C、3F). 以上结果表明,慢性束缚应激会减少小肠各肠段中ZO-1,PCNA蛋白阳性表达量和杯状细胞数量.
-
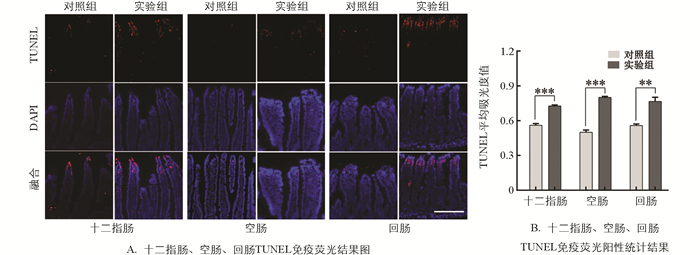
通过TUNEL染色方法检测慢性束缚应激对小鼠肠道细胞凋亡的影响,结果显示与对照组相比(图 4A、4B),实验组小肠组织TUNEL阳性表达显著性升高29.89%,60.81%,37.09%(p<0.01).
-
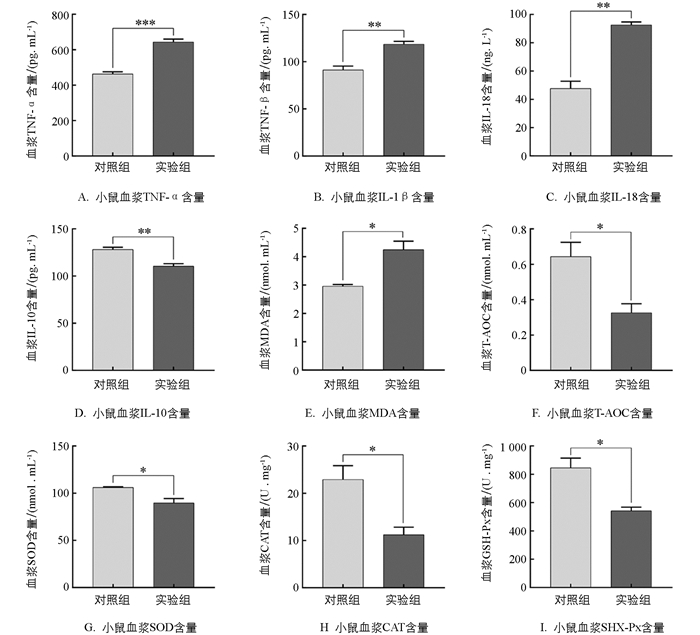
ELISA实验检测显示,实验组炎症因子TNF-α,IL-1β,IL-18含量显著高于对照组39.14%,30.24%,98.21%(p<0.01),而IL-10显著低于对照组13.85%(p<0.01,图 5A、5B、5C、5D). 为了探明应激是否造成小鼠氧化应激发生,本研究检测了小鼠血浆氧化-抗氧化系统变化. 实验结果显示与对照组相比,实验组血浆中MDA含量增加43.44%(p<0.05,图 5E);而T-AOC,SOD,CAT,GSH-Px含量减少49.65%,15.47%,47.75%,35.08%(p<0.05,图 5F、5G、5H、5I). 以上实验结果提示机体处于氧化应激水平,表明慢性束缚应激会导致氧化物质和促炎细胞因子含量升高,抗氧化物、抗炎因子含量降低,致使机体氧化-抗氧化系统失衡,加重机体炎症反应.
2.1. 慢性束缚应激小鼠模型建立验证分析
2.2. 慢性应激对肠道组织结构变化的影响
2.3. 肠道免疫组织化学和PAS染色结果
2.4. 肠道TUNEL染色结果
2.5. 肠道抗氧化能力及炎性细胞因子表达的变化情况
-
本研究对小鼠进行为期24 d慢性束缚应激,同时每天记录各组体质量及采食量数据. 结果发现,应激小鼠体质量较正常组显著降低,但两组采食量差异不具有统计学意义. 造模结束后通过ELISA实验检测到慢性束缚应激小鼠血浆NE和CORT激素水平较对照组显著上升,同时OFT实验结果发现实验组小鼠出现焦虑行为. 岳云双[16]研究证实,慢性不可预知应激可显著提高小鼠血浆NE和CORT水平. 徐小英等[17]关于慢性应激对内分泌系统及肠道微生物菌群的作用研究发现,血浆CORT水平显著增多,肠道也不同程度有损伤. 据此,本研究进一步探索慢性应激对小鼠小肠屏障结构的影响,结果显示应激造成小鼠肠绒毛显著变短,隐窝深度增加,V/C比值显著降低. 小肠发挥作用得益于肠上皮屏障结构完整,紧密连接蛋白则发挥着维持机械屏障完整性和屏障功能的作用. 大量研究表明,一旦紧密连接蛋白结构被破坏,会导致肠道上皮屏障完整性缺陷和慢性炎症[18]. 本研究还通过免疫组织化学方法检测了小肠紧密连接蛋白ZO-1的表达情况,结果发现慢性束缚应激小鼠各肠段紧密连接蛋白ZO-1表达下调,提示肠上皮机械屏障受损. 杯状细胞是肠粘液产生的主要细胞,也是维持上皮结构完整性不可或缺的重要组成. 因此,杯状细胞数量决定粘液分泌多少. 本研究中PAS染色结果显示慢性束缚应激小鼠杯状细胞数量显著减少,提示肠黏膜屏障功能受到抑制. 张淑芳[19]发现束缚应激小鼠结肠组织内杯状细胞数量显著减少、炎症程度增加. 相关研究进一步证明,慢性应激降低肠道sIgA水平,并影响小鼠十二指肠黏膜上皮内淋巴细胞数量[20]. 这与本研究统计的肠内皮单核淋巴细胞数量结果一致,提示慢性应激通过减少内皮细胞单核淋巴细胞数量来破坏肠道免疫屏障.
在生理情况下,小肠上皮处于自我更新、分化、凋亡和环境的平衡. 有证据表明外源因素,如益生菌可通过增强肠细胞分化、增加绒毛高度和绒毛高度与隐窝深度的比率、降低隐窝深度来促进完整绒毛的发育[21]. 为此,本研究通过TUNEL染色法检测了小鼠中肠绒毛顶端凋亡细胞数量,结果显示在慢性应激小鼠中肠绒毛顶端TUNEL阳性表达细胞数量显著高于正常组,与此相反的是PCNA肠隐窝基底层阳性表达显著低于正常组. Del Carmen Martínez-Sánchez等[22]研究发现细胞骨架重排和细胞脱落紊乱会引发通透性增加,导致肠道组织结构的损伤和炎症. PCNA反应细胞增殖情况,可作为细胞增殖活性的可靠标记物,其表达上调或下调提示细胞增殖水平上升或下降. 因此,本研究发现慢性应激妨碍肠道隐窝基底干细胞的增殖分化,同时增加绒毛顶端凋亡细胞,破坏肠道上皮结构,影响肠屏障的完整性.
慢性应激造成小肠机械屏障损伤,而机械屏障损伤可导致病原微生物的入侵,从而致使肠道炎症反应发生[23]. 本研究对小鼠血浆进行ELISA检测发现,束缚应激小鼠TNF-α,IL-1β,IL-18水平显著上调,IL-10含量显著下调. 慢性应激诱发原发性肠炎,TNF-α是体外和体内研究中导致紧密连接蛋白结构被破坏最重要的炎症因子,能够破坏血管内皮结构,显著降低ZO-1表达[24]. IL-1β还可以通过影响紧密连接增加肠道通透性[25]. 在小鼠慢性应激诱导抑郁行为研究中发现,慢性应激增加IL-18释放量[26],它作为一种促炎细胞因子,可通过抑制杯状细胞成熟来抑制粘蛋白产生[27]. IL-10是一种具有抗炎作用的细胞因子,在限制炎症反应和防止组织损伤方面具有关键作用,有研究发现束缚应激诱发和加剧IL-10缺乏小鼠的炎症[28]. 因此,本研究结果表明,慢性束缚应激通过损伤肠道屏障,诱导小鼠炎症反应.
氧化应激是生命中常见的生理过程,其特征是由于线粒体氧化磷酸化反应导致体内多种活性氧(ROS)过量产生[29]. 鉴于小肠对氧化应激的敏感性很高,结合先前研究发现慢性应激增加氧化应激,并诱导十二指肠的肠屏障通透性和细胞凋亡[30],本研究推测机体氧化-抗氧化系统失衡是导致肠屏障损伤的因素之一. 因此,本研究利用生化手段检测了机体氧化-抗氧化相关指标,结果表明在束缚应激小鼠血浆中MDA水平显著上调,相反SOD,CAT,GSH-Px水平和T-AOC显著下调. 有研究发现在抗氧化剂和氧化系统不平衡的情况下,过量的ROS可以通过减少紧密连接和细胞数量来扰乱上皮细胞完整性和肠道屏障[31]. 在关于自由基清除酶的研究中发现SOD,CAT,GSH-Px等抗氧化物酶活性下降不利于自由基清除,自由基和ROS导致机体细胞衰老、脂质过氧化,影响肠上皮自我更新[32]. ROS过量产生会导致脂质过氧化MDA增多、刺激细胞产生促炎细胞因子、诱导细胞凋亡、造成紧密连接结构损伤[33]. 综上所述,慢性束缚应激致使肠道发生氧化应激,导致肠上皮细胞凋亡增加、更新变慢及上皮紧密连接蛋白ZO-1表达下降,加重炎症反应及肠屏障结构完整性破坏.
-
本研究发现慢性束缚应激通过诱导小鼠机体发生氧化应激反应,从而破坏肠道细胞增殖凋亡平衡,进而造成肠道屏障结构完整性受损,引起有害物质进入肠道固有层,诱导肠道和机体炎性反应,导致炎症性肠病发生.






 DownLoad:
DownLoad: