-
开放科学(资源服务)标识码(OSID):

-
犬弓首蛔虫(Toxocara canis,T. canis)是一种广泛分布于世界各地的人兽共患寄生线虫. T. canis生活史复杂,其虫卵随粪便排出后,在适宜的自然环境下发育为感染性虫卵[1-3]. 终末宿主摄入感染性虫卵后,幼虫经循环系统移行至肠道发育为成虫. 非特异宿主摄入感染性虫卵后,幼虫不能发育为成虫,而是移行到神经、肌肉、内脏等组织器官,成为滞育的感染性L3幼虫[4-5]. 人(特别是儿童)因污染的水、土壤、水果和蔬菜中的感染性虫卵或食入来自非特异性宿主如牛、羊、兔、鸡等未煮熟的肉/内脏中滞育的感染性幼虫而感染. 根据侵入器官、感染强度、迁移持续时间及年龄和宿主的免疫反应,可以导致多种临床综合征[6-7]. 此外,感染性虫卵还可引起心肌内膜炎、脑膜炎、眼内炎、哮喘及癫痫等疾病,因此研究犬弓首蛔虫在兽医学及公共卫生学上都具有重要意义.
超氧化物歧化酶(Superoxide Dismutase,SOD)是一种广泛存在于动物、植物和微生物体内的金属酶类[8-10]. 根据辅基所结合的金属离子不同,SOD可分为4种类型,分别为Cu/Zn-SOD,Mn-SOD,Fe-SOD及Ni-SOD[11],只在某些极少数原核细菌中报道有NiSOD存在[12]. SOD是维持抗氧化系统的第一道防线,它能够催化超氧阴离子自由基歧化生成过氧化氢(H2O2)和氧(O2),在保护机体细胞免受氧自由基毒害中起到至关重要的作用,且与很多疾病的发生和发展紧密相关[13]. 寄生虫SOD在抗氧化、免疫逃避和虫体存活等过程中起着重要的作用. 据报道,大片吸虫(Fasciola gigantica)分泌排泄蛋白SOD诱导的抗氧化作用与虫体逃避中性粒细胞、巨噬细胞或树突状细胞产生活性氧(Reactive Oxygen Species,ROS)的免疫反应有关[14];疟原虫(Plasmodium vinckei) SOD通过降解宿主红细胞的血红蛋白产生ROS,以利于红细胞内疟原虫的存活[15];克氏锥虫(Trypanosoma cruzi) MnSOD缺乏会加剧线粒体电子传递链的功能障碍,并造成患病动物心脏的过度氧化损伤[16].
目前,对于犬弓首蛔虫超氧化物歧化酶的研究较少. 本研究运用分子生物学技术,首先克隆Tc-sod基因并进行序列分析;随后构建Tc-sod/pET-32a原核表达载体并制备多克隆抗体,以期为进一步研究Tc-SOD的生物学功能奠定基础.
HTML
-
T.canis虫体采自于西南大学荣昌校区动物医院的患病犬. 虫体经过洗涤及形态学鉴定后冷冻于液氮中. 从T. canis雌虫卵巢收集虫卵,放置于恒温培养箱中培养1~2周获得L2幼虫;新西兰大白兔购自西南医科大学实验动物中心.
PremixTaq DNA聚合酶、pMD19-T (simple)Vector、限制性内切酶XhoⅠ、BamHI、PrimeScriptTM RT reagent Kit购自TaKaRa公司;Trizol试剂购自Invitrogen(赛默飞)公司;HRP标记的山羊抗兔IgG购自Sangon Biotech公司、Ni-NTA纯化树脂预装柱、Protein A+G Agarose抗体纯化试剂盒购自碧云天生物技术公司、EasyPure©胶回收试剂盒、质粒提取试剂盒购自北京TransGen Biotech(全式金)公司.
-
根据T. canis基因组数据(GenBank:AAB00227.1),利用Primer-Premier 6.0对Tc-sod进行引物设计. 基因克隆引物(F1:5'-ATGACAGGATTGTTATTGGC-3';R1:5'-TTACGGTGCGCCTGAGCTC-3')和原核表达引物(F2:5'-CGCGGATCCATGCAAAGACCAAATTCA-3',引入酶切位点BamHI;R2:5'-CCGCTCGAGTTACGGTGCGCCTGAGCT-3',引入酶切位点XhoⅠ),送至重庆擎科兴业生物科技有限公司进行合成.
-
采用TRIzol法提取T.canis雄虫、雌虫和L2幼虫的总RNA,并用核酸蛋白测定仪检测其浓度和纯度;以提取的总RNA为模板,按PrimeScriptTM RT reagent Kit说明书反转录合成cDNA.
-
以反转录的T. canis雄虫、雌虫和L2幼虫cDNA为模板进行PCR扩增. PCR反应条件为:94 ℃预变性5 min、94 ℃变性30 s、56 ℃退火30 s、72 ℃延伸30 s,共35个循环,最后72 ℃延伸10 min,扩增产物经1%琼脂糖凝胶电泳,用凝胶成像系统扫描并记录结果.
按照EasyPure©胶回收试剂盒说明书切胶回收PCR产物,将回收产物与pMD19-T (simple)Vector载体进行连接,全部连接产物转化至100 μL大肠杆菌(Escherichia coli)DH5α感受态细胞中,涂布于含IPTG和X-gal的LB/Amp+琼脂平板中,于37 ℃恒温培养箱正置培养30 min,再倒置培养12~16 h. 挑取单菌落接种于含LB/Amp+液体培养基中,37 ℃、180 r/min培养12~24 h,将菌液PCR鉴定为阳性的重组菌液送至重庆擎科兴业生物技术有限公司测序. 测序结果利用Clustal Omega软件进行多重序列比对;采用MEGA 7.0的邻接法(Neighbour-joining,NJ法)构建系统进化树,并用Bootstrap进行进化树可靠性分析,共1 000个重复.
-
按质粒提取试剂盒说明书提取阳性重组质粒Tc-sod/pMD19-T和pET-32a质粒,经限制性内切酶XhoⅠ和BamHI双酶切,切胶回收目的基因Tc-sod与pET-32a质粒、DNA Ligation Kit进行连接反应. 在冰上配制连接反应液:Digested pET-32a DNA 1 μL、Tc-sod 4 μL、Ligation Mix 5 μL;16 ℃反应1 h. 重组表达质粒转化至DH5α感受态细胞,经蓝白斑筛选后挑取白色单个菌落接种于LB/Amp+液体培养基中,37 ℃ 185 r/min培养12~14 h. 利用北京TransGen Biotech(全式金)质粒小提试剂盒提取质粒,进行PCR、双酶切及测序鉴定.
-
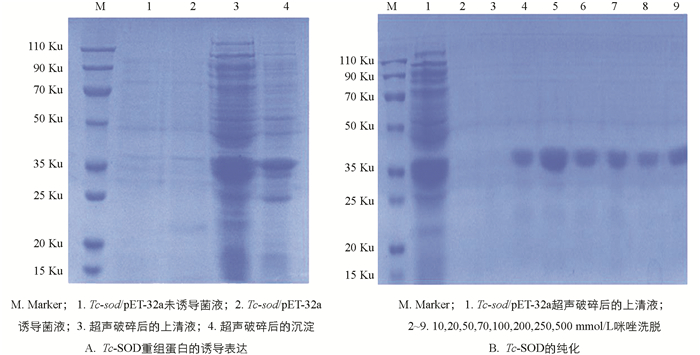
挑选阳性菌,经PCR鉴定后提取质粒,并将其转化至BL21(DE3)感受态细胞,挑单菌落接种于LB/Amp+液体培养基中,37 ℃ 185 r/min振荡培养3 h,OD600值约为0.8,加入0.4 mmol/L的IPTG 37 ℃诱导8 h;将菌液置于15 ℃离心机,4 500 r/min离心10 min,收集菌体沉淀,用PBS洗涤离心2~3次后,再用Washing Buffer重悬菌体,并进行超声破碎、离心,分别取破碎上清液和破碎沉淀进行SDS-PAGE电泳检测;采用Ni-NTA纯化目的蛋白,并选择不同浓度梯度的咪唑洗脱液对目的蛋白进行洗脱,利用SDS-PAGE电泳检测纯化结果.
-
利用透析法去除重组蛋白中的咪唑,对蛋白进行浓缩以达到免疫浓度. 以新西兰大白兔(体质量2~2.5 kg)为免疫动物,首免将250 μg重组蛋白与弗氏完全佐剂1∶1混合,乳化后在颈背部皮下以多点注射的方式对两只健康新西兰大白兔进行首免;初次免疫后每隔2周加强免疫1次,共加强免疫3次. 采用250 μg重组蛋白与弗氏不完全佐剂1∶1混合,乳化后免疫. 末次免疫7 d后采血,离心取血清. 将纯化后的重组蛋白用包被液稀释为5 μg/mL,4包被过夜,利用ELISA法检测抗体效价,酶标仪检测OD450值,当检测孔与阴性孔的比值大于(等于)2.1时的最大稀释倍数为该血清最高的抗体效价. 达到所需效价后采血制备、纯化多克隆抗体,以兔抗Tc-SOD多克隆抗体1∶10 000稀释作为一抗,并用辣根过氧化氢酶(HRP)、标记的羊抗兔IgG(1∶5 000)稀释作为二抗,进行Western Blot检测.
1.1. 材料
1.2. 方法
1.2.1. 引物的设计与合成
1.2.2. 总RNA的提取与反转录
1.2.3. Tc-sod全长基因克隆和序列分析
1.2.4. 原核表达载体的构建
1.2.5. 目的蛋白的表达与纯化
1.2.6. 多克隆抗体的制备及特异性鉴定
-
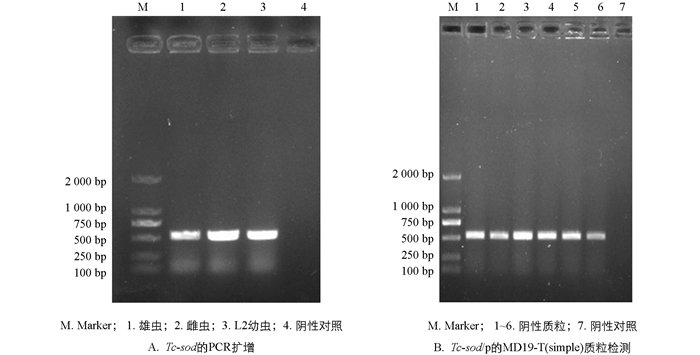
经PCR扩增后1%琼脂糖凝胶电泳结果显示Tc-sod在雄虫、雌虫和L2幼虫的全长CDS(Coding Sequence)序列大小约为573 bp(图 1A). 将凝胶回收产物与pMD19-T(simple)连接,经菌液PCR鉴定后,电泳结果显示在573 bp处有特异性条带,阴性对照没有出现条带(图 1B).
-
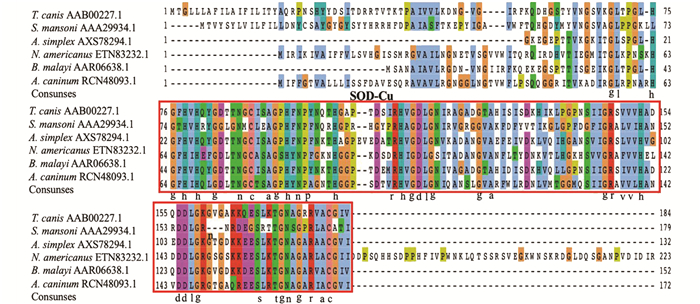
将阳性克隆的菌液进行测序,并对从雄虫、雌虫、L2幼虫获得的碱基序列进行分析,结果显示Tc-sod全长基因的序列长度为573 bp,含有1个完整的CDS,共编码190个氨基酸残基;功能结构域分析结果显示Tc-SOD蛋白的1~19位氨基酸为信号肽区域,含有一个跨膜区,46~184位为SOD-Cu保守结构域. 将测序获得的Tc-SOD氨基酸序列与曼氏血吸虫(Schistosoma mansoni;GenBank NO. AAA29934.1)、异尖线虫(Anisakis simplex;GenBank NO. AXS78294.1)、美洲板口线虫(Necatoramericanus;GenBank NO. ETN83232.1),马来丝虫(Brugia malayi;GenBank NO. AAR06638.1)、犬钩口线虫(Ancylostoma caninum;GenBank NO. RCN48093.1)的SOD蛋白氨基酸序列进行多重序列比对,结果显示均含有SOD-Cu保守结构域(图 2红色方框标记).
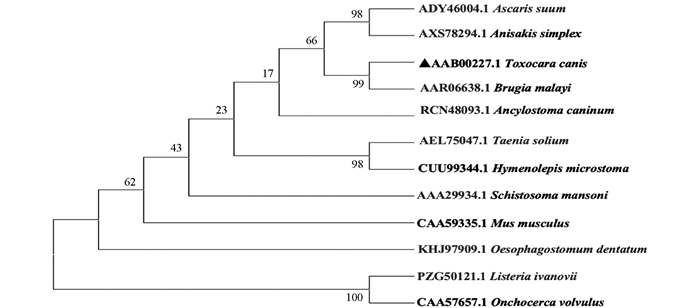
将Tc-sod编码的氨基酸序列与GenBank收录的马来丝虫(B.malayi,GenBank NO. AAR06638.1)、曼氏血吸虫(S.mansoni,GenBank NO. AAA29934.1)、家鼠(Mus musculus,GenBank NO. CAA59335.1)、膜壳绦虫(Hymenolepis microstoma,GenBank NO. CUU99344.1),盘尾丝虫(Onchocerca volvulus,GenBank NO. CAA57657.1)、犬钩口线虫(A.caninum,GenBank NO.RCN48093.1)、李斯特菌(Listeria ivanovii,GenBank NO. PZG50121.1)、猪带绦虫(Taenia solium,GenBank NO.AEL75047.1)、猪蛔虫(Ascaris suum,GenBank NO. ADY46004.1)、异尖线虫(A.simplex,GenBank NO.AXS78294.1)、有齿食道口线虫(Oesophagostomum dentatum,GenBank NO. KHJ97909.1)氨基酸序列进行比对并构建进化树(图 3),结果显示Tc-SOD与马来丝虫(B. malayi,GenBank NO. AAR06638.1)处于同一分支,表明其进化关系较近.
-
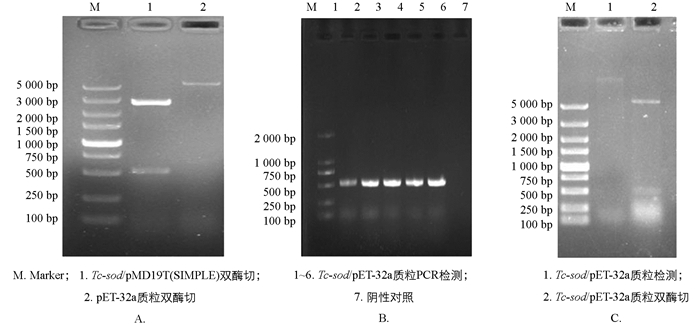
将测序成功的Tc-sod序列亚克隆至pET-32a,经菌液PCR检测,可见1%琼脂凝胶电泳结果具有清晰明亮的条带,且大小与预期相符(图 4B);经双酶切鉴定,结果表明已成功连接至pET-32a(图 4C).
-
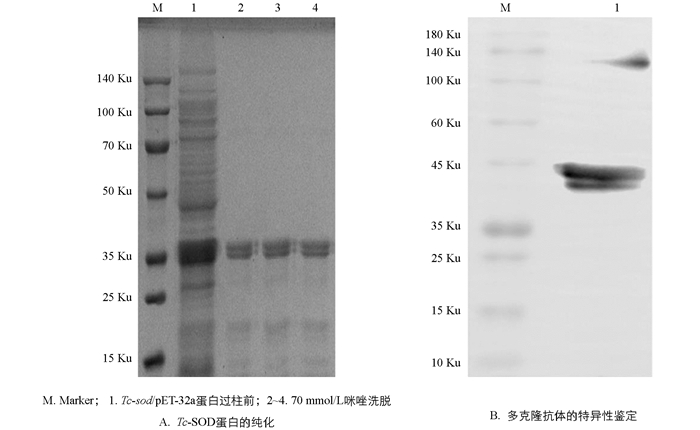
将阳性重组质粒转化至BL21(DE3)感受态细胞后挑取单个菌落培养,于37 ℃经IPTG诱导后进行SDS-PAGE分析,结果显示表达产物大多数都是以可溶性蛋白的形式存在于上清液中,只有少量存在于包涵体内(图 5A);重组蛋白Tc-SOD大小约为37 ku;利用Ni-NTA对重组蛋白进行纯化,结果表明纯化后的蛋白经SDS-PAGE检测无明显杂带(图 5B),蛋白具有较高的浓度和纯度;且在筛选纯化条件时,选择70 mmol/L咪唑洗脱液进行洗脱的蛋白浓度更高,效果更好(图 6A).
-
将纯化后的重组蛋白Tc-SOD包被96孔板,免疫前的血清为阴性对照,利用间接ELISA法测定兔抗Tc-SOD多克隆抗体的效价,结果显示抗体滴度>1∶320 000,具有较高的效价,可用于后续试验. Western Blot检测结果显示兔抗Tc-SOD多克隆抗体较高,能和重组Tc-SOD蛋白特异性结合,表明其具有较好的特异性.
2.1. Tc-sod全长基因的PCR扩增
2.2. Tc-SOD蛋白氨基酸多重序列比对及种系发育进化分析
2.3. Tc-sod/pET-32a表达质粒构建
2.4. Tc-SOD重组蛋白的诱导表达及纯化
2.5. 多克隆抗体的特异性鉴定
-
超氧化物歧化酶(SOD)是生物体内普遍存在的一类重要金属抗氧化酶,在机体氧化与抗氧化平衡中起到至关重要的作用,其经典功能是清除体内超氧阴离子并参与活性氧自由基代谢. 研究表明,SOD与抗氧化、心血管疾病、肿瘤及免疫逃避等密切相关[17-19]. 此外,SOD还参与生物机体的多种生理/病理反应. 在小鼠体内SOD参与调节乳腺发育所需的蛋白质和转录因子对氧化应激的不同敏感反应,被证明与小鼠的乳腺发育及哺乳有关[20]. 在微生物中ROS细胞水平由抗氧化酶和小分子抗氧化剂控制,当细菌被免疫效应细胞吞噬后暴露在氧化应激环境下,细菌将能量从细胞质NADPH转移到抗氧化酶中,从而增强其毒力作用[21].
寄生虫SOD具有抗氧化、免疫逃避和虫体存活等多种生物学功能. 寄生虫在寄生过程中既有宿主作为防御系统产生的ROS,也有虫体代谢产生的ROS,因此清除这双重来源的ROS成为寄生虫能够建立长期寄生的重要前提,而ROS的产生被认为是虫体针对宿主产生免疫逃避的一个重要防御机制. 盘尾丝虫(Onchocerca volvulus)微丝蚴和成虫在人体迁移和寄居过程中通过SOD清除有毒自由基,以利于虫体存活[22]. Lalrinkima等[14]报道大片吸虫SOD诱导的抗氧化作用与虫体逃避巨噬细胞产生活性氧(ROS)的免疫反应有关.
本研究发现犬弓首蛔虫SOD含有保守的SOD-Cu结构域. SOD-Cu作为铜伴侣能介导特定细胞内信号分子的传递途径,其主要功能是将Cu2+输送到Cu/Zn SOD不同细胞内的特定靶点,对其功能的发挥至关重要. Cu2+与酶活性水平也有密切关系. 在哺乳动物中缺乏铜伴侣的小鼠组织SOD基因活性明显减少,对氧化应激敏感程度更高[23]. 铜伴侣在介导氧化应激反应过程中参与激活SOD活性调节机制,通过调节其表达水平以应对氧化环境的变化[24]. Feng等[25]证明甲壳动物的SOD被Cu2+激活后,在免疫调节反应中发挥重要作用.
在重组蛋白表达系统中,大肠杆菌表达系统是目前最为成熟的原核表达系统. 本研究所选择的pET-32a载体表达的目的蛋白带有组氨酸标签,能通过镍柱有效纯化重组蛋白. 对表达条件进行优化,在37 ℃诱导8 h,能得到大量的可溶性重组蛋白;通过镍柱亲和层析纯化,最终在70 mmol/L咪唑浓度洗脱时得到的目的蛋白条带单一,表明重组蛋白具有较高的浓度和纯度. 将纯化后的重组蛋白免疫新西兰大白兔制备多克隆抗体,通过间接ELISA法对制备的抗Tc-SOD多克隆抗体效价及抗原特异性进行鉴定. 结果显示本研究成功制备出了抗Tc-SOD多克隆抗体,效价高达1>320 000. Western Blot检测显示制备的多克隆抗体能够识别Tc-SOD,具有较好的特异性.
-
犬弓首蛔虫Tc-sod基因全长序列为573 bp,共编码190个氨基酸;成功构建了Tc-sod/pET-32a原核表达载体,得到了可溶性重组蛋白,免疫新西兰大白兔并得到了高效价的多克隆抗体;Western Blot检测显示抗体特异性较高. 本试验为后续Tc-SOD的生物学功能研究奠定了基础.






 DownLoad:
DownLoad: