-
开放科学(资源服务)标识码(OSID):

-
睡莲(Nymphaea spp.)是睡莲科(Nymphaeaceae)睡莲属(Nymphaea)多年生浮叶水生观赏草本的统称,50多个种或变种广布在从热带到寒带的各个区域,按其生态型主要分为热带睡莲和耐寒睡莲[1-2]. 睡莲因其多样的花香、花色和独特的花型,广泛应用于园林水景、室内外花卉装饰、鲜切花等. 睡莲生长发育对氮、磷等需求量大,根系发达,对水体中的一些重金属如铬、镍有较强的吸附能力,在水体生态修复方面具有较突出的优势[3-4]. 睡莲根茎可入药作为麻醉剂、收敛剂、抗炎剂等治疗疾病,一些种类的花、嫩叶、叶柄可用于餐饮[5-8]. 睡莲属于基部被子植物,是研究植物进化的重要材料[9-10]. 睡莲栽培历史悠久,尤其是在西方文明中被视为圣洁的象征[11]. 因此,睡莲集观赏、生态、药用、食用、科研和文化价值于一身,成为水生观赏植物研究的重点和热点.
花色是观赏植物最重要的性状之一[12],决定了观赏植物的价值. 睡莲的花色不但有常见的红、白、黄、紫色系,还有其他植物稀缺的蓝色系,这使其在蓝色花育种方面备受关注[2, 10-11, 13]. 关于睡莲的花色研究,已有报道解析了常见的热带和耐寒睡莲花瓣类黄酮含量、组分与花色的关系,综述了睡莲花色研究进展,为睡莲花色育种研究提供了有力支撑[2, 10-11, 13-20]. 类黄酮是植物花色的主要呈色物质之一,主要包括花青苷、黄酮醇苷、黄酮类等. 在类黄酮生物合成途径中,编码查尔酮合成酶(CHS)、查尔酮异构酶(CHI)、黄烷酮3-羟化酶(F3H)、类黄酮3′-羟化酶(F3′H)、类黄酮3′5′-羟化酶(F3′5′H)、黄酮醇合成酶(FLS)、二氢黄酮醇4-还原酶(DFR)、花青素合成酶(ANS)、糖基转移酶(GT)等类黄酮生物合成的核心结构基因,同时,调控这些结构基因的转录因子MYB,bHLH和WD40也起到了重要作用[21-22]. 因此,研究植物花瓣中类黄酮的组分和含量、花色遗传规律、生物合成途径以及花色关键基因及其功能,解析花色形成的物质基础、分子机理和基因调控网络,可为培育花色新品种提供理论参考[2, 23]. 在花色分子育种方面,对类黄酮合成途径相关基因进行遗传调控是实现花色改良的主要途径[22],如利用基因工程技术进行花色改良培育出了蓝色月季(Rosa chinensis)和菊花(Chrysanthemum×morifolium)新品种[24-25].
近年来,随着对睡莲花色形成分子机理的深入研究,目前已鉴定了睡莲类黄酮代谢途径的一些关键结构基因如F3H[26],GT6[27]和R2R3型MYB转录因子基因MYB6[28],并进行了功能分析. 但是关于蓝星睡莲(N. colorata)的类黄酮成分和含量以及花色发育关键基因的深入分析与挖掘,还鲜见报道. 蓝星睡莲属于广热带亚属睡莲,有本种蓝色花和天然突变体白色花两种花色,基因组小,为2倍体,是研究睡莲蓝色花形成的理想模式材料[10]. 本研究以蓝、白两种花色的蓝星睡莲为试材,解析不同花发育时期花瓣类黄酮成分和含量,同时对类黄酮生物合成途径核心基因进行实时定量PCR(RT-qPCR)分析,鉴定蓝星睡莲蓝色花形成和蓝白花色差异的关键基因,为进一步阐明蓝星睡莲类黄酮生物合成机制奠定基础,同时也为睡莲蓝色花分子育种提供理论依据.
HTML
-
供试蓝花和白花蓝星睡莲种球购于南京艺莲苑,种植于西南大学水产学院水生植物资源圃. 材料采样时间为7-8月,分别采集5个发育时期的花瓣. 花瓣的5个发育时期划分以花瓣着色程度为依据,S1:未着色的花瓣;S2:花瓣中部以上着色;S3:花瓣全部呈紫红色;S4:花瓣全部为蓝紫色但未开放;S5:花开放第1天. 将试验材料置于液氮中速冻后放于-80 ℃超低温冰箱中保存备用.
-
新鲜蓝星睡莲花瓣经液氮研磨后采用提取液提取花青苷和黄酮醇苷,提取方法参照Wu等[13]的方法. 花青苷和黄酮醇苷含量采用Agilent 1260(Agilent,美国)测定. 梯度洗脱程序为:流动相A为0.1%甲酸,流动相B为乙腈;0 min 5% B,6 min 45%B,9 min 90% B,9.1 min 10% B,13 min 10% B,13.5 min 5% B,20 min 5% B. 流速0.5 mL/min,进样量为7 μL. 色谱柱为Agilent TC-C18 column(5 mm,4.6 mm × 250 mm),柱温35 ℃,样品室温度10 ℃. 以矢车菊素3-O-葡萄糖苷(Cyanidin 3-O-glucoside,Cy3G)和芦丁(Rutin)作为标准品. 以每克鲜质量(FW)中含有的毫克数为单位计算,3个生物学重复,使用SPSS 20.0对数据进行Turkey方差分析,显著性分析水平为p<0.05.
花青苷和黄酮醇苷成分采用UPLC-Triple-TOF/MS系统. AcquityTM ultra型高效液相色谱仪(Waters,美国)和Triple TOF 5600+型飞行时间质谱以及电喷雾离子源(AB SCIEX,美国) 系统进行测定,具体方法参考文献[22]. 仅梯度洗脱程序稍加改动:流动相A为0.1%甲酸,流动相B为乙腈;0 min 5% B,6 min 45%B,7 min 90% B,7.1 min 10% B,10 min 10% B,10.2 min 5% B,13 min 5% B. 流速0.4 mL/min,进样量为7 μL. 花青苷和黄酮醇苷物质通过质谱结果,结合软件数据库搜索、出峰顺序以及对应参考文献[5, 10, 13-17, 20]进行成分鉴定.
-
蓝色、白色蓝星睡莲5个发育时期的花瓣经液氮充分研磨后,使用Omega Plant RNA Kit(Omega,美国)提取Total RNA. 获得的Total RNA经质检合格后,采用反转录试剂PrimeScript RT reagent Kit with gDNA Eraser Kit(Takara,日本)进行反转录,获得相应的cDNA后分别稀释5倍后用于荧光定量PCR试验.
-
分别以类黄酮生物合成途径相关基因(CHS,CHI,F3H,FLS,F3′H,F3′5′H,DFR,ANS,GT,MYB1,bHLH1,WD40)和内参基因NcACT11的RT-qPCR引物(表 1),分析它们在蓝星睡莲不同花发育时期(S1~S5)的转录表达情况. RT-qPCR采用ABI QuantStudioTM 3 Real-Time PCR Systems(ABI,美国)测定. 荧光染料为NovoStart© SYBR qPCR Super Mix Plus(novoprotein,中国),反应体系和反应程序按说明书进行. 试验设置3个生物学重复,用2-ΔΔCT方法计算基因的相对表达量,使用SPSS 20.0对数据进行Turkey方差分析,显著性分析水平为p<0.05.
1.1. 试验材料
1.2. 蓝星睡莲不同发育时期花青苷和黄酮醇含量与成分的测定
1.3. 蓝星睡莲不同发育时期总RNA提取及cDNA合成
1.4. 蓝星睡莲花色形成过程中相关基因表达分析
-
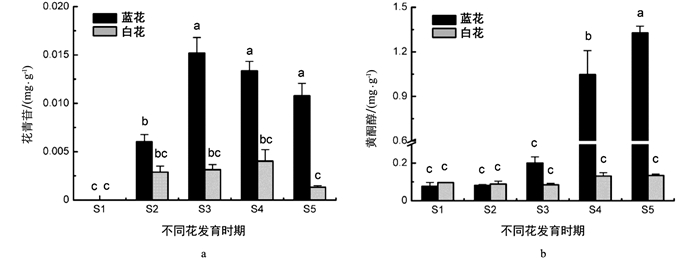
采用高效液相色谱法对两种花色蓝星睡莲5个发育时期花瓣(S1~S5,图 1)的花青苷、黄酮醇苷含量进行了测定. 结果表明,蓝、白花瓣花青苷和黄酮醇苷在各个时期的成分一致. 蓝、白蓝星睡莲花瓣除在S1时期无花青苷积累,其他时期均检出花青苷,其含量均呈现出先升高后降低的趋势(图 2a). 具体地讲,在未着色的S1时期未检测到花青苷,随着花发育进程,花青苷在S2时期开始积累,花瓣呈蓝紫色;花青苷从顶部逐渐向基部扩展,蓝花花瓣花青苷含量在S3时期达到最大值(0.015 mg/g FW),花色仍为蓝紫色;在S4和S5时期,花青苷含量逐渐降低,花瓣呈蓝色. 蓝花花瓣花青苷含量在S3~S5时期无显著性差异,但与其他时期均有显著性差异. 白花花瓣花青苷含量在S4时期达到最大值,仅0.040 mg/g,5个时期的花青苷含量无显著性差异. 在着色的花瓣中,除S2时期,蓝花花瓣花青苷含量均显著高于白花.
同时测定了两种颜色花瓣黄酮醇苷含量,结果表明,随着花发育进程,蓝星睡莲蓝花花瓣黄酮醇苷含量呈先缓慢升高后快速上升的趋势,在S5时期达到最大值(1.327 mg/g),与其他时期花瓣黄酮醇苷含量均有显著性差异. 而白花花瓣黄酮醇苷含量在5个花发育时期变化不大,含量均在0.100 mg/g左右浮动. 蓝、白花瓣的黄酮醇苷含量在S1,S2时期相当,但在S3时期前者比后者增加了1倍,到S4,S5时期,蓝花花瓣黄酮醇苷含量与白花的均呈现出显著性差异,在S5时期,蓝花是白花的10倍(图 2b).
-
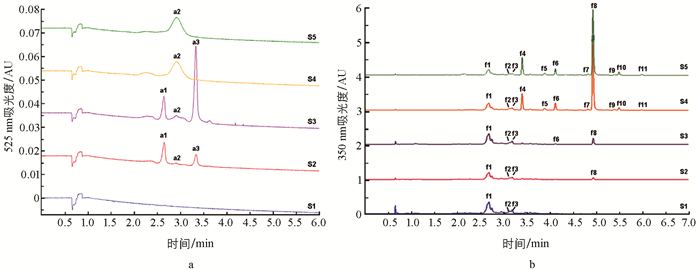
通过UPLC-Triple-TOF/MS系统测定了花瓣花青苷和黄酮醇苷的主要成分,在正离子模式下,共推定了蓝星睡莲花瓣中的花青苷主要有3种(图 3a,表 2,a1~a3). 花瓣在S1时期无花青苷积累,在S2,S3时期主要呈色花青苷为a1飞燕草素-3-O-β-半乳糖苷(delphinidin 3-O-β-galactopyranoside,Dp3Ga),其次为a3飞燕草素-3-O-(2″-O-没食子酰-6″-O-乙酰-β-半乳糖苷)(delphinidin 3-O-(2″-O-galloyl-6″-O-acetyl-β-galactopyranoside),Dp3galloyl-acetyl-Ga);在S4和S5时期,花青苷a1和a3含量明显减少,主要呈色花青苷为a2飞燕草素-3′-O-(2″-O-没食子酰-6″-O-乙酰-β-半乳糖苷)(delphinidin 3′-O-(2″-O-galloyl-6″-O-acetyl-β-galactopyranoside),Dp3′galloyl-acetyl-Ga)(图 3a,表 2).
在负离子模式下,在蓝星睡莲花瓣中共鉴定出11种黄酮醇苷(图 3b,表 2,f1~f11),主要为杨梅素、槲皮素和山奈酚的衍生物. 组分f1~f3在所有时期的花瓣中均存在,其中组分f1槲皮素3-O-半乳糖苷(quercetin 3-O-galactoside,Qu3Ga)是S1~S3时期的主要黄酮醇苷. 随着花发育进程,其他组分也逐渐被检出. 在花发育后期S4,S5时期,其主要黄酮醇苷为组分f8杨梅素3-O-α-L-(3″-O-丙二酰)-鼠李糖苷(myricetin 3-O-α-L-(3″-O-malonyl)-rhamnopyranoside,My3malonyl-Rh),其次是组分f4杨梅素3-O-α-L-鼠李糖甘(myricetin 3-O-α-L-rhamnopyranoside,My3Rh)、组分f6杨梅素3-O-α-L-(3″-O-乙酰)-鼠李糖苷(myricetin 3-O-α-L-(3″-O-acetyl)-rhamnopyranoside,My3acetyl-Rh)、组分f1 Qu3Ga、f10槲皮素3-O-α-L-(3″-O-丙二酰)-鼠李糖苷(quercetin 3-O-α-L-(3″-O-malonyl)-rhamnopyranoside,Qu3malonyl-Rh).
-
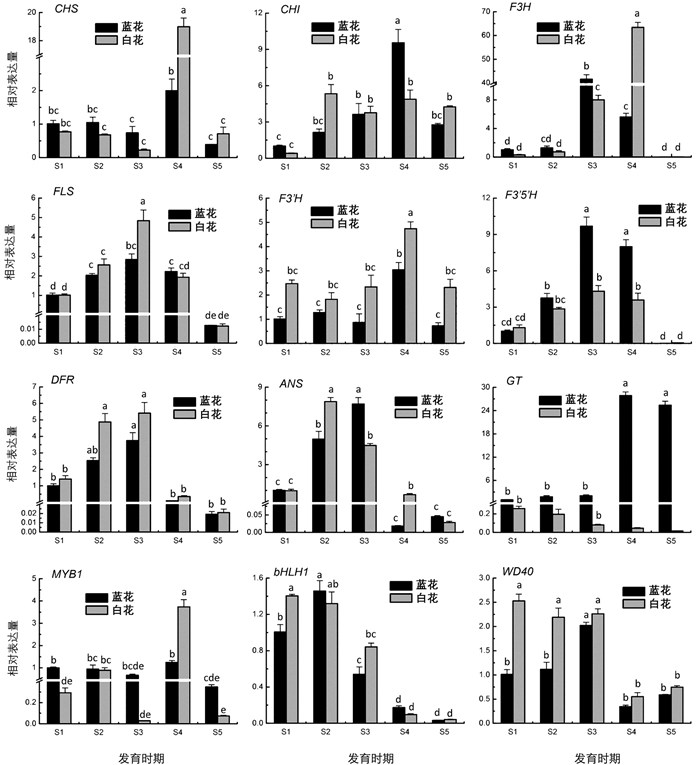
为了探索蓝星睡莲花青苷和黄酮醇苷含量变化与物质合成途径相关基因表达的关系,依据本研究花青苷和黄酮醇苷分析结果与课题组前期转录组数据,本研究选择了类黄酮生物合成途径中的编码核心酶和调控蛋白的基因进行转录表达分析. 结果表明,F3′5′H,FLS,DFR随着蓝色花瓣花发育进程均呈现出逐渐升高,在S3时期达到峰值,然后下降的趋势,与其花青苷积累变化趋势相似. 此外,F3′5′H和GT在蓝色蓝星睡莲花瓣中的表达量均高于白色种,F3′5′H和GT分别在蓝色花瓣S3,S4时期和S4,S5时期的表达量显著高于其他时期,而F3′H,DFR则在白色花瓣中的表达量略高于蓝色种(图 4).
2.1. 蓝星睡莲不同发育时期花瓣花青苷和黄酮醇苷含量
2.2. 蓝星睡莲不同发育时期花青苷和黄酮醇苷成分分析
2.3. RT-qPCR分析蓝星睡莲不同发育时期类黄酮合成途径基因的表达变化
-
花瓣的呈色是多因素综合的结果,其中色素种类和含量是最主要的因素[12, 22-23, 29]. 本研究中,蓝星睡莲不同花发育时期花色出现变化,在S2~S3时期花瓣呈蓝紫色,主要呈色花青苷为Dp3Ga,其次为Dp3galloyl-acetyl-Ga;在S4~S5时期花瓣呈蓝色,主要呈色花青苷为Dp3′galloyl-acetyl-Ga. Zhu等[15]认为热带睡莲花瓣呈蓝紫色主要是因为飞燕草素3位被酰基化糖苷修饰,而呈蓝色则是飞燕草素3′位发生酰基化糖苷修饰. 在S2,S3时期,正是由于飞燕草素3位发生了糖苷和酰基化糖苷的修饰,导致花瓣主要呈蓝紫色;在S4,S5时期,主要是飞燕草素3′位发生酰化糖苷修饰,而使花瓣主要呈蓝色. 这与朱满兰[30]对35种热带睡莲花青苷成分的分析结果一致,该研究结果认为热带蓝色系睡莲大部分只有Dp3Ga,少数品种以Dp3′Ga为主,Dp3Ga次之,而蓝星睡莲属于这其中的少数品种.
本研究在蓝星睡莲花瓣中只检出了1种花青素苷元即飞燕草素,但其衍生物花青苷有3种,其中S4,S5时期的主要呈色物质为Dp3′galloyl-acetyl-Ga,与先前报道的结果一致[10]. 本研究细化了蓝星睡莲花色发育过程,在该物种中检测到了另外2种花青苷物质Dp3Ga和Dp3galloyl-acetyl-Ga,它们在其他热带睡莲中也有报道[13, 15]. 蓝星睡莲花瓣黄酮醇苷以杨梅苷为主,达6种;其次是槲皮苷,共4种;只检测到了1种山奈酚苷. 朱满兰[30]认为,在热带蓝色系睡莲中,绝大多数品种以槲皮苷型色素为主,少数以山奈酚苷为主,极少部分以杨梅苷为主;而本研究中,在花瓣发育过程中,黄酮醇苷的主要成分由前期以槲皮苷为主逐渐转变为后期以杨梅苷为主,这说明共色素黄酮醇苷的种类和含量也随花色发育进程而发生变化.
在类黄酮含量方面,蓝星睡莲花瓣着色从顶端开始向基部蔓延,蓝花花青苷积累随花发育进程出现先稳步上升后逐渐降低的趋势,在S3~S5时期显著高于白花品种;在含量差异最大的S3时期,蓝花花瓣花青苷含量比白花高约50倍. 黄酮醇苷作为花青苷的共色素,可以提升植物的显色[31]. 本研究中,蓝星睡莲蓝花花瓣黄酮醇含量随花发育进程逐渐增加,其中,在S4,S5时期急剧增加,显著高于同时期的白花花瓣. 在蓝花花瓣中,花青苷和黄酮醇苷含量和主要呈色物质随发育进程而出现变化,从而导致花色也随之改变,而在白花花瓣中,虽在S2~S5时期检测到了花青苷,但含量显著低于蓝花;此外,白花花瓣中的黄酮醇苷含量在5个花发育时期变化不大,在0.100 mg/g附近轻微浮动,尤其是在S4~S5时期,含量远低于蓝花花瓣. 因此,花青苷成分变化是引起蓝花花色变化的直接原因,而花青苷含量的巨大差异是造成蓝、白花色差异的主要原因,黄酮醇苷含量差异加剧了这种花色差异.
研究表明,CHS,CHI,F3H,FLS,F3′H,F3′5′H,DFR,ANS,GT这一系列催化酶和转录复合物MYB-bHLH-WD40在类黄酮合成路径中发挥重要作用[21-22]. 本研究中,通过RT-qPCR分析编码这些酶的结构基因和调控基因在蓝、白色花瓣中的表达情况,结果发现,随着花发育进程的推进,F3′5′H,FLS,DFR表达变化与蓝色花瓣花青苷积累变化趋势一致,推测其可能是蓝色花形成的关键基因. 通过对蓝星睡莲MYB1-bHLH1-WD40转录复合物进行转录表达分析,发现它们与花色发育关系不大,可能不是影响蓝星睡莲花色发育的调控因子,具体的关键调控因子还有待进一步挖掘. 吴倩[27]和田洁[28]推定GT6和MYB6是“泰国王”睡莲花色形成的关键结构基因和转录因子. 在葡萄风信子亚美尼亚(Muscari armeniacum)蓝色花发育过程中,R2R3-MYB转录因子基因MaAN2与MaDFR和MaANS协同表达,且与花瓣花青苷积累趋势一致,表明它们可能是葡萄风信子花瓣花色发育的关键基因[32],进一步的研究表明转录因子MaMybA,MaAN2与MabHLH1互作靶向MaDFR和MaANS,协作调控蓝色花瓣花青苷的积累[33].
Zhang等[10]通过对蓝星睡莲盛开的(等同于本研究中的S5时期)蓝白花瓣转录组测序,推测ANS和GT可能是造成蓝白花色差异的关键基因. 本研究通过动态检测类黄酮合成途径中关键基因在不同发育时期的蓝白花瓣中的表达,结果表明,ANS在蓝白花瓣各时期的表达差异不明显,而F3′5′H和GT在蓝花中的表达量均高于白花,DFR和F3′H则刚好相反,推测在蓝花中表达上调的F3′5′H将底物向飞燕草素方向分流,且GT将不稳定的花青素转变为稳定的花青苷;而在白花中上调的DFR将底物向无色花青素方向催化,F3′H将底物向黄酮醇方向催化,且GT在白花中低表达,导致白花花青苷和黄酮醇苷积累减弱,花色减淡. Liu等[34]通过研究不同花色毛茛的基因表达情况发现,红色花品种中F3H,F3′H,3GT,5GT和FMT2(类黄酮-O-甲基转移酶2基因)上调表达,而紫色花品种中,F3′5′H和3MAT(花色苷3-O-葡萄糖苷-6-O-丙二酰基转移酶基因)转录水平升高,说明不同花色毛茛中的高表达基因不同. 葡萄风信子亚美尼亚蓝白花色差异的主要原因是白色葡萄风信子DFR低表达和FLS高表达,FLS强势竞争底物导致白色葡萄风信子无法合成飞燕草素[35]. 由此可见,植物花色丰富,花色形成差异的原因也因物种不同而不同. 因此,只有有针对性地解析不同物种花色形成的机理,总结花色发育规律,才能更好地为花色分子育种提供可靠的理论参考.







 DownLoad:
DownLoad: