-
开放科学(资源服务)标识码(OSID):

-
硝化作用是全球氮素循环的基本过程,在土壤生态系统的氮素转化过程中起着关键作用。土壤中进行的硝化作用是由微生物主导的两步过程:包括氨氧化微生物主导的氨氧化过程和亚硝化氧化菌主导的亚硝酸盐氧化过程[1-2]。直到2015年,在硝化螺菌属谱系Ⅱ(Nitrospira lineage Ⅱ)中发现了具备完成氨氧化和亚硝酸盐氧化能力的微生物,打破了由两种微生物来分别完成两步硝化过程的传统观念[3],因此被命名为全程氨氧化细菌(complete ammonia oxidizer,Comammox)[4-5],只能进行氨氧化反应的AOA、AOB则被称为半程氨氧化微生物(incomplete ammonia oxidizers)[4, 6]。根据编码氨单加氧酶基因α亚基(amoA)的系统发育分析,Comammox可被分为两个分支:clade A和clade B[4, 7],clade A则可进一步划分为clade A.1、A.2和A.3[8]。研究表明Comammox广泛存在于土壤生态系统中[9-10],可能在土壤硝化作用中起着重要的作用,但目前仅从水生生态系统中培育得到了归属于clade A的纯培养物Nitrospira inopinata[4],尚未从土壤中获得代表性富集物或分离物[11],这限制了我们对Comammox在土壤硝化作用中的理解。
环境因素是影响微生物地理分异规律的重要驱动力[12],土地利用方式同时受到人为因素及环境因素的干扰,对土壤微环境、土壤理化性质及栖息微生物都会造成影响[13]。例如耕作措施、施肥方式及水分管理等差异,会改变土壤性质(如土壤养分、通气状况等),而土壤条件的差异则会进一步影响硝化微生物的丰度及活性[14-15]。稻田是一种独特的人为水生生态系统,由于施用大量的氮肥及长期进行淹水管理,其土壤内部通气状况差,常形成缺氧环境[16]。林地受到的人为干扰较小,土壤养分含量较低。有研究表明,不同土地利用方式所形成的土壤环境差异推动了参与氨氧化过程的功能微生物AOA和AOB的群落组成和生态位分化[14, 17-18],而不同土地利用方式下全程氨氧化细菌与半程氨氧化微生物的分布规律仍不完全清楚。本研究选用由同一母质发育的林地、旱地和水田土壤作为研究对象,分别测定其土壤的硝化活性,通过定量PCR技术分析3种不同土地利用方式下AOA、AOB和Comammox的菌群丰度及活性,利用克隆测序分析Comammox群落的结构变化,旨在进一步了解土地利用方式对全程氨氧化细菌及半程氨氧化微生物的影响。
HTML
-
紫色土发育的水田及旱地土壤采自重庆市北碚区西南大学国家紫色土土壤肥力与肥料效益检测基地(30° 26′ 36″ N,106° 26′ 33″ E)。该区域为亚热带季风气候,年均温18.2 ℃,年均降水量1 105 mm,供试土壤为紫色土,由侏罗系沙溪庙组紫色泥岩风化的残积、坡积物发育而成;林地土壤采自邻近区域相同成土母质发育的土壤(人为干扰少)。3种土地利用方式植被覆盖差异:水田主要进行水稻—大麦轮作,旱地以喜旱莲子草(42%)和石龙芮(35%)为主,林地则主要生长攀倒甑(15%)及构树(10%)。
-
土壤样品采集于2019年5月,在每个采样地随机选择3个间隔10 m以上的5 m×5 m样方进行采样,每个采样点以直径为6.5 cm的土钻按照5点取样法采集0~20 cm深度的土壤样品,混合均匀后低温保存带回实验室。土样剔除树根、其他侵入体及较大石砾后分为3份,第1份鲜土储存在4 ℃冰箱中1周内测定土壤硝化势及亚硝酸盐氧化势;第2份土样自然风干至不粘手时研磨通过2 mm筛,储存于-20 ℃冰箱中用于提取土壤DNA;第3份土壤样品完全风干后研磨通过1 mm筛用于测定土壤基本理化性质,每个土壤样品3次重复。
-
硝化势是指示好氧氨氧化微生物硝化活性的重要指标,对土壤样品进行一段时间培养后,测定土壤样品中硝化过程产生的NO3--N含量,计算单位时间内单位土壤(干质量)所形成NO3--N的量。具体步骤按照鲁如坤[19]所述稍作修改:将15 g新鲜土壤加入100 mL含NH4+的培养液中[培养液组成:1.5 mL 0.2 mol/L KH2PO4、3.5 L 0.2 mol/L K2HPO4、15 mL 0.05 mol/L (NH4)2SO4均加入到1 L容量瓶中用纯水定容],制备土壤悬浮液,经过24 h振荡培养(200 r/min,25 ℃),NH4+被氧化为NO3-;分别在第2、4、22、24 h采集10 mL悬浮液,用10 mL 2 mol/L KCl溶液浸提土壤悬浮液后振荡静置于室温,测定土壤中NO3--N含量。
亚硝酸盐氧化势按Attard等[20]所描述的进行:将5 g新鲜的土壤样品(每克干土含25μg NO2--N)和50 mL NaNO2振荡培养24 h(200 r/min,25 ℃),分别在第2、4、22、24、30 h取样,采集4 mL土壤悬浮液,用4 mL 2 mol/L KCl溶液浸提,将其振荡2 h(200 r/min,25 ℃)后静置30 min,测定土壤中NO2--N含量。土壤NO2--N的含量随培养时间呈线性减少,斜率即可作为土壤亚硝酸盐氧化势。
-
按照制造商的说明方法,采用Power Soil DNA Isolation Kit(MoBio Laboratories,USA)试剂盒提取土壤DNA并分别通过NanoDrop 2000分光光度法和1%琼脂糖凝胶电泳法检测土壤DNA的纯度和完整性。
-
使用实时荧光定量PCR仪(Quant Studio TM 6 Flex)测定AOA、AOB、clade A、clade B amoA基因的拷贝数。clade A、clade B amoA基因的引物序列参照Pjevac等[7]所述。实时荧光定量PCR的总反应体系为20 μL,包括DNA模板1 μL,上游引物、下游引物以及ROX Reference DyeII(50×)各0.4 μL,Taq DNA聚合酶10 μL及无菌水7.8 μL。qPCR反应扩增条件与王梅等[21]描述一致,每个样品进行3个生物学重复。本研究qPCR分析的扩增效率为96%~105%,R2值为0.996~0.999。clade A、clade B amoA基因克隆测序由上海凌恩生物科技有限公司完成,每个样品挑选50个阳性克隆。
-
利用SPSS 26.0软件对土壤硝化势,亚硝酸盐氧化势,AOA、AOB、clade A、clade B amoA基因拷贝数进行统计分析,采用Pearson法进行氨氧化微生物相对丰度与土壤硝化活性之间的相关性分析,利用Origin 8.6软件作图。clade A和clade B克隆测序得到的OTU序列在NCBI的GenBank数据库中进行BLAST比对,获得高度相近的同源基因序列,再采用MEGA11.0软件在97%的相似度下对所有的序列进行操作分类单元(Operating Taxonomic Unit,OTU)聚类,最后采用邻接法(Neighbor-Joining,NJ)构建系统发育树。
1.1. 研究区域概况
1.2. 土壤取样与准备
1.3. 土壤硝化势及亚硝酸盐氧化势的测定
1.4. 土壤微生物总DNA的提取
1.5. 定量PCR及克隆测序
1.6. 数据分析
-
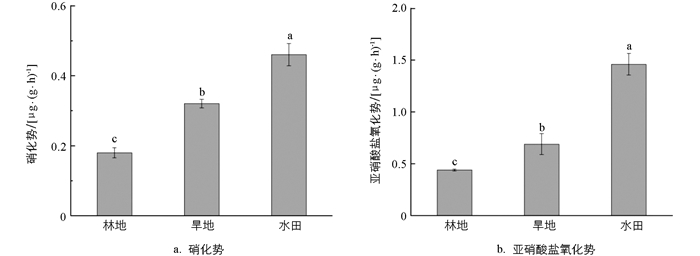
由图 1a可知,不同土地利用方式下的土壤硝化势差异有统计学意义(p<0.05),硝化势的变化范围为0.18~0.46 μg/(g·h),其中水田土壤具有最高的硝化势,为0.46 μg /(g·h);旱地其次,为0.32 μg/(g·h);林地的硝化势最低,为0.18 μg/(g·h)。由图 1b可知,不同土地利用方式下的亚硝酸盐氧化势差异有统计学意义(p<0.05),其中水田具有最高的亚硝酸盐氧化势,为1.46 μg/(g·h),分别是旱地[0.69 μg /(g·h)]和林地[0.44 μg /(g·h)]的2.1和3.3倍。以上结果表明,水田土壤更有利于硝化作用的进行,而林地土壤硝化潜力最弱。
-
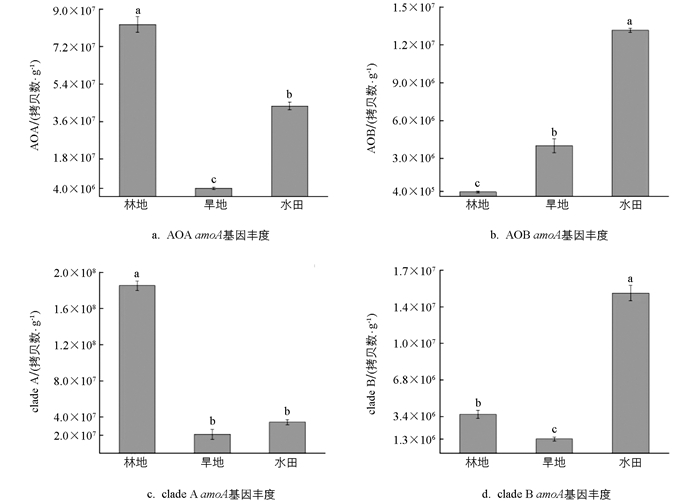
定量PCR分析比较了紫色土发育的3种不同土地利用方式下土壤AOA、AOB、clade A、clade B amoA基因的丰度,结果显示紫色土的不同土地利用方式造成了土壤氨氧化微生物相对丰度的差异。由图 2a可知,紫色土发育而来的林地土壤AOA amoA基因的丰度显著高于其他土壤(p<0.05),达到8.26×107拷贝数/g;在水田土壤中AOA amoA基因的丰度显著降低了47.34%,为4.35×107拷贝数/g;旱地土壤丰度最低,为4.01×106拷贝数/g,表明林地土壤更有利于AOA的生长。由图 2b可知,水田土壤AOB amoA基因的丰度达到最大值,为1.38×107拷贝数/g,分别是林地和旱地土壤的38.4倍和3.4倍,差异有统计学意义(p<0.05)。由图 2c可知,紫色土发育而来的3种土地利用方式下clade A amoA基因的丰度差异有统计学意义(p<0.05),林地clade A amoA基因的丰度为1.85×108拷贝数/g,比旱地(2.10×107拷贝数/g)和水田(3.44×107拷贝数/g)高出1个数量级,clade A与AOA相似,都在林地土壤中达到丰度最大值。由图 2d可知,水田的clade B amoA基因的丰度为1.48×107拷贝数/g,林地次之(3.94×106拷贝数/g),旱地丰度最低,为1.24×106拷贝数/g(p<0.05)。
由表 1可知,土地利用方式显著影响了土壤中AOA/AOB和clade A/ clade B amoA基因丰度的比率。具体而言,林地土壤AOA/AOB amoA基因比率最高,达到229.3,而水田和旱地AOA/AOB amoA基因比率分别为3.1和1.1,两者差异不大,说明在利用氨方面,AOA具有更好的氨亲和力,更适应于低营养的林地土壤环境。与AOA/AOB amoA基因比率结果类似,3种不同土地利用方式下的clade A amoA基因的丰度都高于clade B,其比率分别为47.0、16.8和2.3,表明在3种土壤中全程氨氧化细菌都以clade A为主;并且与AOA丰度相似,clade A在林地土壤中丰度最高,而水田环境中全程氨氧化细菌的不同分支分布更加均匀。
-
Pearson相关性分析结果表明,氨氧化微生物的丰度与土壤硝化活性显著相关(表 2)。clade A与土壤硝化势呈显著负相关(R=-0.719,p<0.05),而与土壤亚硝酸盐氧化势无显著相关性。clade B与亚硝酸盐氧化势呈显著正相关(R=0.671,p<0.05),与土壤硝化势无显著相关性。AOA与硝化势及亚硝酸盐氧化势无显著相关性。AOB与土壤硝化势及亚硝酸盐氧化势均呈极显著正相关(R1=0.955,R2=0.891,p<0.01)。氨氧化微生物的丰度之间也具有相关性,其中clade A与AOA呈显著正相关(R=0.776,p<0.05),与AOB呈极显著负相关(R=-0.799,p<0.01)。clade B与AOA呈显著正相关(R=0.669,p<0.05)。
-
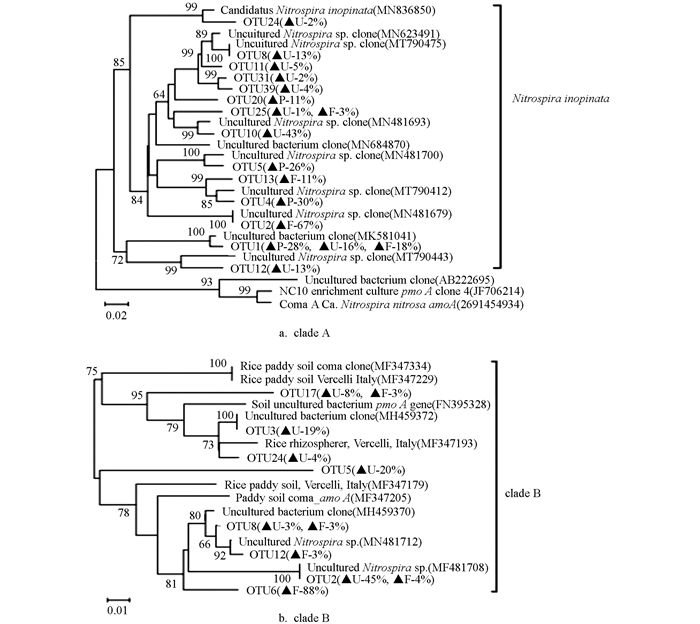
将不同土地利用方式土壤下的Comammox进行克隆测序分析,并构建系统发育树,如图 3。
结果表明紫色土发育的林地、旱地和水田土壤的Comammox群落结构组成均与Nitrospira inopinata物种密切相关,未检测到Nitrospira nitrosa物种,但是不同土地利用方式下土壤OTU占比存在显著差异。clade A系统发育分析总共得到14个OTU,林地土壤共有4个OTU,其中OTU2占比最大,为67%;旱地土壤共得到9个OTU,其中OTU10占比最大,为43%;水田土壤得到4个OTU,其中OTU4占比最大,为30%,其次是OTU1(28%),OTU5(26%)和OTU20(11%)。clade B系统发育分析总共得到8个OTU,林地土壤共得到5个OTU,其中OTU6占比最高(88%),与序列MF347205相关;旱地土壤所涉及的OTU最为广泛,为6个OTU,其中OTU2占比最高,为45%;水田土壤中未发现clade B的OTU。
2.1. 不同土地利用方式下土壤的硝化势和亚硝酸盐氧化势
2.2. 不同土地利用方式下土壤氨氧化微生物的丰度
2.3. 土壤硝化活性与氨氧化微生物基因丰度的相关性
2.4. 全程氨氧化细菌的系统发育分析
-
本研究的结果显示,土地利用方式对全程及半程氨氧化微生物的丰度及硝化活性有显著影响(p<0.05),不同土地利用方式导致的氨有效性[3, 22]、水分[11, 17]及氧气有效性[23-24]的差异可能是导致全程及半程氨氧化微生物在不同土地利用类型的土壤中发生生态位分化的主要原因。
Comammox及半程氨氧化微生物AOA、AOB都以NH3为氮源进行自养生长,并且在不同土壤环境中广泛分布共存。已有的研究确定氨的有效性是造成AOA和AOB之间生态位分化的一个重要因素[25-26]。在氮素含量较低的林地土壤中,AOA的丰度显著高于大量施用氮肥的水田,并且林地土壤AOA丰度比AOB高出2个数量级,而AOB在水田中达到丰度最大值(图 2),表明AOA在低氮的贫营养环境中更具有竞争优势,AOB则更适应富氮土壤生境。Dimitri等[3]对Comammox Nitrospira inopinata的氨氧化动力学分析结果显示,其氨亲和力高,可能比半程氨氧化微生物更适应贫营养的生存环境。在低氨浓度的水生生态系统[27]及生物过滤器[28]的研究中表明,Comammox Nitrospira inopinata在贫营养环境中发挥着重要作用。在本研究中,林地中占优势的氨氧化微生物为clade A,其丰度分别是半程氨氧化微生物AOA和AOB的2.2倍和47.0倍,表明底物有效性是导致硝化微生物类群生态位分化的重要因素。在氨限制的贫营养土壤环境中,Comammox Nitrospira inopinata比半程氨氧化微生物更具竞争优势。研究指出clade B具有amt型氨转运蛋白序列,与clade A具有的rh型转运蛋白序列相比具有更高的氨亲和力[24],这一区别可能是造成clade A与clade B之间生态位分化的一个重要原因。本研究结果显示,在3种不同土地利用方式中clade A的丰度均高于clade B,这与Xu等[29]对全国采集的典型农业土壤研究中得出的结果相一致,表明虽然底物浓度是影响clade A和clade B之间生态位分化的一个因素,但仍存在其他环境因素的综合作用推动紫色土中两者的生态位分化,并且在紫色土中,全程氨氧化细菌以clade A为主。
氨氧化过程是硝化过程的第一步也是限速步骤,需要氧分子的参与才能完成氨的活化。Santoro等[30]对半程氨氧化微生物AOA和AOB的研究指出,AOB丰度随着氧气浓度的降低而减少,而AOA比AOB更适宜于低氧环境。对Comammox纯培养物的基因组分析结果表明,Comammox Nitrospira inopinata可能具有潜在的低氧耐受特性[4, 24],且多生存于低氧浓度的环境中[24]。此外,在低氧浓度下运行的生物反应器中,Comammox的高度富集也表明在微氧环境条件下,Comammox比半程氨氧化微生物更具有竞争优势[23],因此,氧的有效性可能是直接调节Comammox、AOA和AOB在土壤环境中分布格局的一个因素[31]。与林地和旱地相比,水田由于长期处于淹水状态,氧气的转移受到限制[32],土壤环境处于缺氧状态。本研究发现,紫色土的clade A均属于Nitrospira inopinata类群(图 3),虽然在水田与旱地中clade A的丰度无显著差异,但水田中clade A的丰度是旱地的1.6倍,在水田中clade B的丰度显著高于林地与旱地(图 2,p<0.05),进一步证明了Comammox对微氧土壤环境的耐受性。在低氧的水田土壤环境中,Comammox两个分支的丰度均高于AOB,这与先前对氧气含量较低的沿海水域及三峡库区河流沉积物中得到的Comammox amoA基因丰度高于AOB的研究结果类似[7, 33],表明氧气含量可能是造成全程与半程氨氧化微生物生态位分化的因素之一。
土壤水分对微生物代谢活动及硝化过程同样起着重要作用[34]。不同土地利用方式所造成的水分状况差异会改变土壤的微生态环境[35-36],例如土壤水分的增加会降低气体的扩散速率,直接影响微生物的活性[37]。增加土壤水分能加快液体的扩散速度,为硝化微生物提供关键底物[37-38]。Dai等[17]指出,水分条件可能是导致旱地与稻田土壤AOA和AOB生态位分化的主要原因,其中AOA更适应于淹水环境,而AOB则在旱地土壤中主导土壤氨氧化过程。在本研究中,AOA丰度从林地到水田显著降低(p<0.05),而AOB则对土壤水分增加产生了积极的响应,这与刘若萱等[39]对不同水分梯度的土壤模拟实验得出AOB对水分变化敏感的结论一致。水田环境同样刺激了clade B的活性,并且clade A与clade B的丰度比值也从林地的47.0降低到水田的2.3,表明clade B可能在缺氧的淹水环境中具有生理活性。Wang等[11]研究中也得到clade B在酸性和中性水稻土的硝化作用中有贡献。在旱地和水田处理中,clade A的丰度并未表现出显著差异,表明在紫色土中clade A可能对土壤水分变化不敏感。
硝化势是指示好氧氨氧化微生物硝化活性的重要指标,亚硝酸盐氧化势则是全程氨氧化细菌和亚硝酸盐氧化菌活性的反映。在本研究中,Pearson相关性分析结果表明,AOB和clade A与硝化势呈显著相关性(p<0.05),AOB和clade B与亚硝酸盐氧化势呈显著相关性(p<0.05),说明这3类氨氧化微生物可能在紫色土的硝化过程中发挥重要作用。相比之下,AOA与土壤硝化活性并无明显相关,表明AOA可能在不同土地利用方式的紫色土中硝化活性较弱,对硝化作用贡献不高,因此不同土地利用方式可能会造成氨氧化微生物的变化,进而影响土壤硝化、亚硝酸盐氧化过程。在本研究中,水田土壤的硝化活性显著高于林地和旱地土壤(图 1),而在林地土壤中全程及半程氨氧化微生物的总丰度高于旱地和水田土壤,但其土壤亚硝酸盐氧化势却显著低于旱地和水田土壤(图 1,p<0.05),进一步表明土壤环境中微生物功能基因的数量并不一定能反映其功能活性[9, 40]。Bai等[41]对不同耕作制度黑土的研究中也指出,虽然AOA与Comammox在丰度上占有很大比重,但是AOB才是促进耕地黑土硝化作用的主要贡献者。
土壤微生物群落结构与土壤环境密切相关,一方面土壤环境的改变会影响土壤微生物的群落结构及其功能;另一方面土壤微生物的结构及功能活性也会影响土壤中各种养分的循环转化过程[42]。本研究发现,不同土地利用方式的紫色土中clade A均归属于Nitrospira inopinata,表明紫色土环境中clade A的系统发育多样性较单一,但不同土地利用方式下土壤中优势Comammox存在差异性,说明不同土地利用方式仍会影响紫色土Comammox的群落结构。Medriano等[43]对不同土地利用类型的热带土壤研究中也得出,人为土地利用导致了热带土壤微生物组成及多样性的改变。
此外,植被组成在调节微生物群落和功能方面发挥着重要作用[44-47]。Subbarao等[48]研究指出胡枝子对硝化作用的抑制机制是通过向土壤释放根系分泌物来阻断细菌的氨氧化途径,这可能也从另一个角度解释了本研究中3种土地利用方式下全程及半程硝化微生物丰度的差异。在本研究中,3种土地生长的植被类型存在差异:林地主要生长攀倒甑(15%)及构树(10%),旱地以喜旱莲子草(42%)和石龙芮(35%)为主,水田则主要进行水稻—大麦轮作,后续研究可从植被类型对土壤硝化功能微生物菌群的影响方面加以考虑。
-
本研究表明,Comammox、AOA和AOB在不同土地利用方式的紫色土中均广泛分布。土地利用方式是影响全程氨氧化细菌(Comammox)及半程氨氧化微生物(AOA和AOB)生态位分化的关键因子。此外,土地利用方式也显著影响Comammox两个分支(clade A和clade B)的生态位分化特征。该研究结果对农业和林业生产种植具有重要指导意义,有助于根据不同土地利用方式优化氮肥管理,提升土壤氮素利用效率,促进作物和林木的健康生长。






 DownLoad:
DownLoad: