-
乙型病毒性肝炎是由乙型肝炎病毒(hepatitis B virus,简称HBV)引起的一种传染病. 乙肝可分为急性乙型肝炎和慢性乙型肝炎,慢性乙肝可导致肝硬化和肝癌甚至死亡[1]. 病毒通过接触感染者的血液或其他体液传播. HBV入侵人体时,通过与受体结合的方式进入肝细胞,然后HBV的DNA被运输到肝细胞的细胞核中进行复制并排出病毒粒子[2-3].
数学模型在研究和控制HBV传播中发挥着不可或缺的作用. 自文献[4]提出一个分析抗病毒治疗对减少病原体载量影响的动力学模型后,许多学者从不同角度建立并分析了HBV动力学模型. 文献[5-8]研究治疗对于减少病毒传播和抑制病毒传播的作用. 文献[9-12]分别研究免疫反应(包括细胞溶解免疫和非细胞溶解免疫)对控制病毒传播的影响. 文献[13]考虑了治疗和已感染细胞的恢复项. 然而,文献[13]没有考虑感染细胞刺激产生CTL免疫细胞所需时间对动力学分析的影响,因此,本文同时考虑了药物治疗和免疫时滞,建立如下模型:
其中:x(t),y(t),v(t)和z(t)分别表示未感染细胞浓度、感染细胞的浓度、游离病毒浓度和CTL细胞的浓度;Λ表示未感染细胞的产生率;σ表示未感染细胞和感染细胞的死亡率;β表示感染率;u1表示药物治疗阻断新感染发生的效率;p表示感染肝细胞通过非细胞溶解机制转移到未感染细胞的恢复率;q表示感染细胞通过免疫介导被杀死的清除率;m表示游离病毒的产生速率;u2表示药物治疗抑制病毒产生的效率;μ表示游离病毒的死亡率;k表示CTL细胞的死亡率;s表示被感染细胞刺激产生CTL细胞的激活率;τ表示细胞免疫时滞.
HTML
-
设X=C([-τ,0],
$\mathbb{R}_ + ^4$ )是[-τ,0]到$\mathbb{R}_ + ^4$ 上的连续映射全体构成的Banach空间,且定义范数‖φ‖=$\mathop {\max }\limits_{ - \tau \le \theta \le 0} $ -τ≤θ≤0 |φ(θ)|,φ∈X. 系统(1)的初值条件如下:其中:φ=(φ1(θ),φ2(θ),φ3(θ),φ4(θ))∈X,φi(θ)≥0,φi(0)>0,i=1,2,3,4. 根据泛函微分方程的基本理论,可知系统(1)满足初值条件(2)有唯一解.
定理1 设(x(t),y(t),v(t),z(t))是系统(1)的满足初始条件(2)的唯一解,则对任意t>0,解是正的且有界的.
证 首先,证明y(t)是正的. 假设结论不成立,即存在t>0,使得y(t)=0. 令t1=inf{t:t>0,y(t)=0},因为y(0)>0,故y′(t1)≤0. 根据系统(1)的第三个方程,有y′(t1)=(1-u1)βx(t1)v(t1). 下面证明y′(t1)>0. 由系统(1)的第一个和第三个方程有:
可知x(t1)>0,v(t1)>0,故y′(t1)>0,矛盾. 故y(t)为正. 易得x(t),v(t)均为正. 下证z(t)为正. 假设结论不成立,即存在t>0,使得z(t)=0. 令t2=inf{t:t>0,z(t)=0},则z′(t2)≤0,z(t2)=0. 由τ>0,t2-τ < t2,得z(t2-τ)>0,又因y(t2-τ)>0,可得z′(t2)=sy(t2-τ)z(t2-τ)>0,矛盾. 故z(t)为正.
下证系统(1)解的有界性. 令
沿着系统(1)对t求导
其中
$\tilde n = \min \left\{ {\frac{\sigma }{2}, \mu , k} \right\}$ . 由比较定理可得其中ε是任意小的正常数. 故系统(1)的解是有界的.
-
利用下一代矩阵法[14],可得系统(1)的基本再生数和免疫再生数:
下面我们考虑平衡点的存在性,令系统(1)右边4个方程为0,可得:
显然,系统(1)存在无病平衡点E0=(x0,0,0,0)=
$\left( {\frac{\mathit{\Lambda }}{\sigma }, 0, 0, 0} \right)$ . 当R0>1时,存在无免疫平衡点E1=(x1,y1,v1,0),其中当R1>1时,存在地方病平衡点E2=(x2,y2,v2,z2),其中
为了简化免疫再生数R1,定义R1*=
$\frac{{\left( {1 - {u_1}} \right)\left( {1 - {u_2}} \right)\beta ms\mathit{\Lambda }}}{{\sigma \mu s(p + \sigma ) + \left( {1 - {u_1}} \right)\left( {1 - {u_2}} \right)\beta m\sigma k}}$ ,有如下引理成立:引理1 (a) R1 < 1⇔R1* < 1;
(b) R1>1⇔R1*>1;
(c) R1=1⇔R1*=1.
证 先证明(a)成立:
类似地,(b)和(c)也成立.
-
定理2 当R0 < 1时,无病平衡点E0局部渐近稳定;当R0>1时,E0不稳定.
证 系统(1)线性化后在E0处的特征方程为
显然方程(3)有两个负特征根λ=-σ,λ=-k. 其它特征根λ满足方程
显然μ+p+σ>0,当R0 < 1时,
故方程(4)的两个特征根实部均为负. 因此,当R0 < 1时,无病平衡点E0局部渐近稳定. 当R0>1时,方程(4)的两个特征根实部一正一负,故E0不稳定.
定理3 当R0≤1时,无病平衡点E0全局渐近稳定.
证 定义Lyapunov泛函如下:
沿着系统(1)轨线的全导数为:
当R0≤1时,V′1≤0. V′1=0当且仅当(x,y,v,z)=E0. 因此,{E0}是{(x,y,v,z)|V′1(t)=0}的最大不变集. 根据LaSalle不变集原理,可知E0全局渐近稳定.
-
定理4 当R1 < 1 < R0时,无免疫平衡点E1局部渐近稳定;当R1>1时,E1不稳定.
证 系统(1)线性化后在E1处的特征方程为
其中
对于方程
当R0>1时,y1>0,故K1,K2,K3均大于0.
由Hurwitz判据法可知方程(6)的特征根均有负实部. 下面考虑方程λ+k-sy1e-λτ=0.
1) 当τ=0时,λ=sy1-k. 定义函数
f(x)对于x求导,有
当R0>1时,(1-u1)(1-u2)βmΛ>μσ(p+σ)>σμp,故对所有的x>0有f′(x) < 0,函数f(x)单调递减. 当
${R_1} = \frac{{\left( {1 - {u_1}} \right)\left( {1 - {u_2}} \right)\beta m}}{{\mu (p + \sigma )}}{x_2} = \frac{{{x_2}}}{{{x_1}}} < 1$ ,有x2 < x1. 结合f(x)单调递减,可知y1=f(x1) < f(x2)=y2,则λ=sy1-k < sy2-k=0. 因此当τ=0时,若R1 < 1 < R0,无免疫平衡点E1局部渐近稳定.2) 当τ>0时,假设方程λ+k-sy1e-λτ=0有纯虚根λ=±iω(ω>0). 代入并分离实部和虚部有
等式两边分别平方再求和有
由1)可知,当R1 < 1 < R0时,sy1-k < 0,这与(7)式矛盾. 故方程λ+k-sy1e-λτ=0的特征值有负实部. 因此,当τ>0时,若R1 < 1 < R0,方程(5)的特征根均有负实部,故无免疫平衡点E1局部渐近稳定.
当R1>1时,R1= x2 x1 >1,即x2>x1,故y2 < y1. 设P(λ)=λ+k-sy1e-λτ,P(0)=k-sy1 < k-sy2=0,
$\mathop {\lim }\limits_{\lambda \to + \infty } P(\lambda ) > 0$ . 可知方程P(λ)=0的特征根有正实部,此时E1不稳定.定理5 当RH < 1 < R0 < 1+
$\frac{\sigma }{p}$ 时,无免疫平衡点E1全局渐近稳定.证 定义Lyapunov泛函如下:
沿着系统(1) 轨线的全导数为:
其中
可知RH < 1 < R0 < 1+
$\frac{\sigma }{p}$ 时V′2≤0. V′2=0当且仅当(x,y,v,z)=E1. 因此,E1是{(x,y,v,z)| V′2(t)=0}的最大不变集. 根据LaSalle不变集原理,E1全局渐近稳定. -
系统(1)线性化后在E2处的特征方程为
其中
当τ=0时,(8)式变为
其中
当R1>1时,ai>0,i=1,2,3,4.
根据Routh-Hurwitz判据知当a1a2a3-a32-a12a4>0时,方程(9)的特征根均有负实部. 根据以上分析,有如下结论:
定理6 当τ=0,R1>1时,若a1a2a3-a32-a12a4>0,系统(1)的地方病平衡点E2局部渐近稳定.
现在我们考虑当τ>0时,纯虚根的存在性. 假设方程(8)有纯虚根iω(ω>0),代入到方程(8)中有
分离实部和虚部有
两边分别平方再求和,可得
其中
定义函数
因此,如果方程(8)有纯虚根iω(ω>0),那么方程
有正实根ω2.
假设方程(10)有m(1≤m≤4)个正实根,分别表示为xn(1≤n≤m),我们有
解出
其中:1≤n≤m,j=0,1,2,…. 设方程(8)的特征根是λ(τ)=α(τ)+iω(τ),满足α(τn(j))=0,ω(τn(j))=ω.
为了讨论系统(1)的Hopf分支存在性问题,有如下定理:
定理7
${\left. {\frac{{{\rm{d}}\alpha _n^{(j)}}}{{{\rm{d}}\tau }}} \right|_{\tau = \tau _n^{(j)}}}{\rm{与}}{\left. {\frac{{{\rm{d}}F}}{{{\rm{d}}\tau }}} \right|_{x = {x_n}}}$ 有相同的符号.证 方程(8)两边对τ求导,可得
因此有
显然
${\left. {{{\left. {\frac{{{\rm{d}}\alpha _n^{(j)}}}{{{\rm{d}}\tau }}} \right|}_{\tau = \tau _n^{(j)}}}与\frac{{{\rm{d}}F}}{{{\rm{d}}\tau }}} \right|_{x = {x_n}}}$ 有相同的符号.由Hopf分支理论,我们有如下定理:
定理8 1) 当(10)式无正实根时,对任意的τ≥0,地方病平衡点E2局部渐近稳定;
2) 当(10)式有正实根时,对于τ∈[0,τ0),地方病平衡点E2局部渐近稳定,其中τ0=min{τn(j)|1≤n≤m,j=0,1,2,…};
3) 当(10)式有正实根时,系统(1)在τ=τ0处产生Hopf分支.
证 1) 结论显然成立.
2) 根据τ0的定义可知,当τ∈[0,τ0)时,(10)式没有正实根,即特征根没有穿过虚轴. 所以对所有的τ∈[0,τ0),(8) 式的特征根都具有负实部,此时E2局部渐近稳定.
3) 因为x0是方程(10) 的单根,可知F′(x0)≠0. 由定理7可知
${\left. {\frac{{{\rm{d}}\alpha }}{{{\rm{d}}\tau }}} \right|_{\tau = {\tau _0}}} \ne 0$ . 假设${\left. {\frac{{{\rm{d}}\alpha }}{{{\rm{d}}\tau }}} \right|_{\tau = {\tau _0}}} < 0$ ,当τ=τ0-ε(ε为任意小的正数)时,方程(8)的特征根有正实部,这与2)是矛盾的. 故${\left. {\frac{{{\rm{d}}\alpha }}{{{\rm{d}}\tau }}} \right|_{\tau = {\tau _0}}} > 0$ ,且当τ=τ0时,方程(8)的特征根实部为0或负. 这意味着系统(1)产生Hopf分支.
3.1. 无病平衡点的稳定性
3.2. 无免疫平衡点的稳定性
3.3. 地方病平衡点的稳定性
-
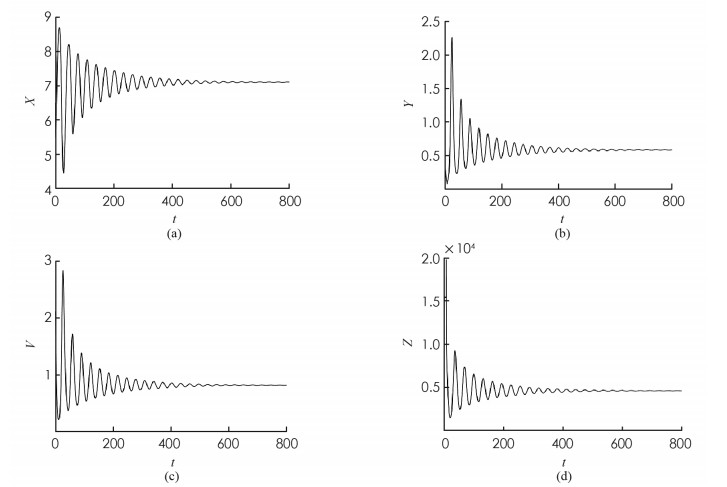
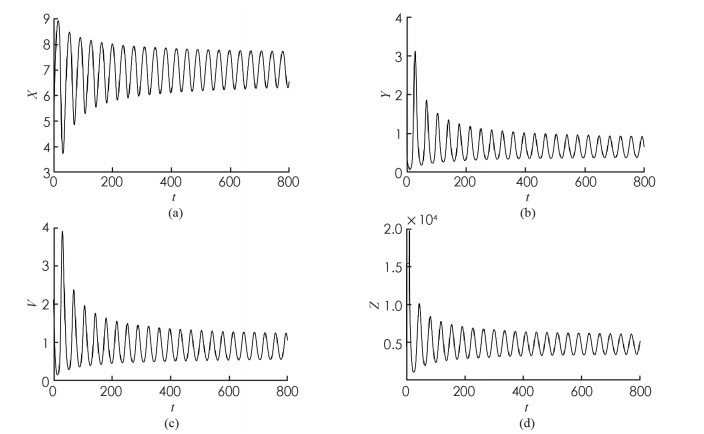
为了验证本文的理论结果,取文献[13,15-16]中的数据:Λ=10;β=4.430 1;p=0.12;σ=1.056 1;q=0.000 7;m=5.352 5;μ=0.381 8;s=9.212 9;k=5.375 2. 令u1=u2=0.9. 可计算出R1=3.748 9,地方病平衡点E2存在且局部渐近稳定,E2=(7.099 3,0.583 4,0.817 9,461.86),τ0=9.704 3.
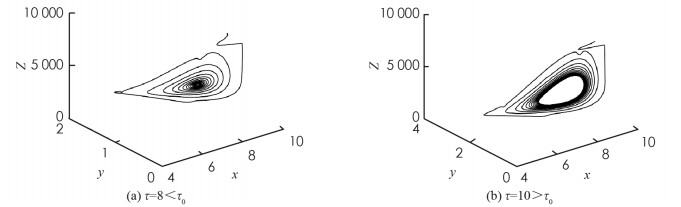
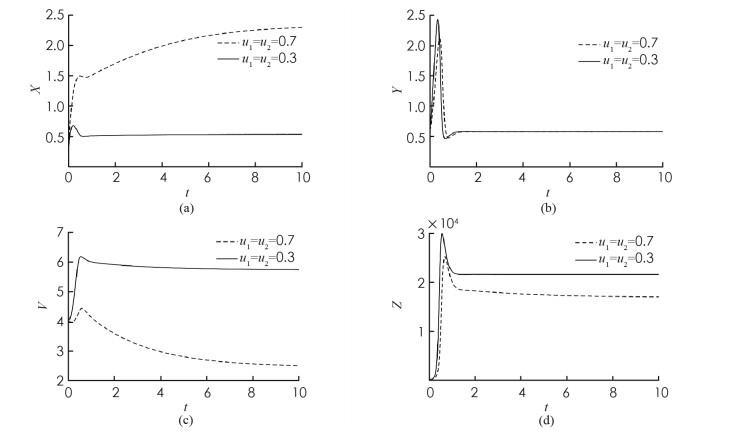
首先,考虑解的长期行为. 当τ=8 < τ0时,地方病平衡点局部渐近稳定(图 1);当τ=10>τ0时,地方病平衡点不稳定并出现了周期解(图 2);图 3展示τ=8和τ=10时的相图. 数值结果与理论结果相一致. 其次,为了探究药物治疗策略对于控制病毒传播的影响,分别取u1=u2=0.7和u1=u2=0.3. 图 4中(a)表明治疗效率越高,未感染细胞浓度的平衡态会明显增加;(b)表明对于不同的治疗效率,虽然感染细胞浓度最终平衡态相同,但是药物治疗效率越高,感染细胞浓度的峰值会有效下降;(c)表明治疗效率越高,自由病毒粒子浓度明显降低;(d)表示治疗效率对降低CTL免疫细胞浓度的影响.
-
本文研究了一个具有药物治疗和CTL免疫时滞的HBV模型,其中药物治疗包括抑制病毒感染和减少自由病毒粒子释放,免疫包括细胞溶解免疫和非细胞溶解免疫. 计算两个阈值:基本再生数R0和免疫再生数R1. 应用LaSalle不变集原理和构造Lyapunov泛函得到3个平衡点的稳定性. 当R0 < 1时,无病平衡点全局渐近稳定;当RH < 1 < R0 < 1+
$\frac{\sigma }{p}$ 时,无免疫平衡点全局渐近稳定. 通过理论分析和数值模拟结果可以看出药物治疗的效率对减少感染细胞(y)和自由病毒粒子(v)浓度有显著效果. 其次,时滞的大小影响地方病平衡点的稳定性:当τ∈[0,τ0)时,地方病平衡点局部渐近稳定;当τ>τ0时,地方病平衡点不稳定. 当τ=τ0时,系统产生Hopf分支. 定理5的RH < 1 < R0 < 1+$\frac{\sigma }{p}$ 作为E1全局稳定成立的条件,或许可以被削弱;另外,本文未得出地方病平衡点E2稳定性的具体结论,今后可进一步讨论.






 DownLoad:
DownLoad: