-
油菜作为重要的油料作物,不仅是食用油与饲料蛋白的主要来源,同时在工业上的应用也非常广泛,因此亟需提高油菜产量,以满足日益增长的需求.在农业生产中,杂种优势因能显著提高作物产量和改善作物品质被广泛应用,其中细胞核雄性不育因具有不育性稳定彻底、无胞质效应等优点,现已成为提高水稻、玉米、油菜等作物产量的重要途径.前人研究表明,致使雄性不育的过程极其繁杂,其中涉及到雄蕊形态建成、花粉囊中各组织分化等多形式、多过程的基因调控,具体调控机理尚未明确.
显性细胞核雄性不育可根据基因发生突变位点及数量不同,将其分为单基因控制的显性核不育、一对复等位基因控制的显性核不育和双基因控制的显性核不育3种不育类型.对D3AB进行遗传分析和验证,认为其是由双基因控制的显性核不育类型.在植物的生命过程中,花粉和花药的发育至关重要,期间涉及多种基因互作[1-2].例如基因AMS[3]以及水稻(Oryza Sativa)中的同源基因TDR都参与绒毡层及小孢子的发育过程,TPD1[4],DYT1[5]及Ms1[6]都参与绒毡层及小孢子的分化.目前,油菜中已发现影响绒毡层细胞发育的相关基因.曾芳琴[7]报道的基因BnCYP704B1如果发生突变,会影响绒毡层细胞中脂类物质的分泌.夏秀云[8]研究的基因BnATA02如果发生突变,会导致绒毡层细胞异常肥大.杨莉芳等[9]综合前人的研究结果,将油菜不育类型分为花粉囊发育异常、减数分裂异常、胼胝质代谢异常、绒毡层发育异常、花粉壁发育异常及花药开裂异常6种类型.
本研究对显性核不育两型系材料D3AB通过体视镜观察花器官的形态特征,采用扫描电镜观察花药发育特点,最后通过石蜡切片观察,确定其发生败育的时期及特点.为深入研究D3A的不育机理奠定基础,同时为进一步推进油菜的杂种优势利用提供理论依据.
HTML
-
实验材料是重庆市油菜工程技术研究中心选育的甘蓝型油菜显性细胞核雄性不育纯合两型系D3AB,不育材料D3A(MsMsrfrf)和可育材料D3B(MsMsRfrf)在花期呈现出明显的表型差异.研究材料D3AB种植于重庆市北碚区西南大学歇马试验基地,种植期间进行常规田间管理,在初花期分别取不育材料D3A和可育材料D3B的花蕾进行后续细胞学研究.
-
在初花期,分别取D3A和D3B不同发育时期的花蕾及盛开花朵置于冰盒中,进行分类整理后按顺序整齐固定摆放至载物台上进行观察拍照,然后依次将这些花蕾的萼片剥离进行观察拍照,最后再将其花瓣剥离至花蕊裸露并进行观察拍照,同时对盛开花朵进行观察拍照.
-
分别取D3A和D3B不同发育时期的花蕾材料进行雄蕊剥离,然后依次将这些雄蕊摆放于贴有专用导电胶的扫描电镜载物台上,并将其固定于扫描电镜样品舱中,进行观察拍照(分别对正、反面进行观察).
-
当油菜进入初花期后,在上午9:00-11:00分别取D3A和D3B不同时期的花蕾立即浸入FAA固定液(50%无水乙醇:冰醋酸:甲醛=90:5:5)中,抽真空30 min(若仍有花蕾漂浮,可重复抽真空操作),进行48 h以上的固定处理,然后依次用百分浓度为50%,70%,90%,100%的乙醇进行梯度脱水,继续进行透明、浸蜡、包埋、切片、展片、脱蜡、复水等处理[10],并用1%甲苯胺蓝溶液染色2~3 min,最后用二甲苯-中性树胶(1:1)进行封片.将制作好的切片利用光学显微镜Nikon E600进行观察拍照.
1.1. 实验材料
1.2. 实验方法
1.2.1. 体视镜样品的制备与观察
1.2.2. 扫描电镜样品的制备与观察
1.2.3. 石蜡切片的制备与观察
-
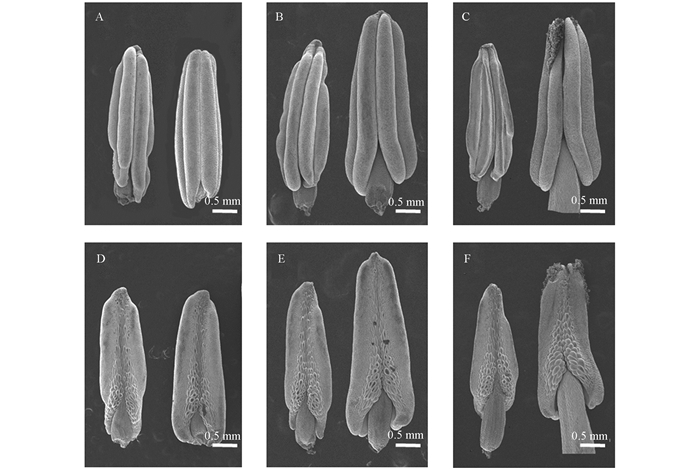
体视镜观察发现(图 1),在花蕾发育的整个过程中,D3A与D3B外观形态上并未呈现出明显差异(图 1A-D),花蕾发育后期,D3B比D3A更加饱满.将不同时期花蕾的萼片及花瓣进行剥离处理后观察发现,在花蕾发育早期,D3A与D3B的雄蕊、雌蕊等花器官均表现正常,但在花蕾发育后期,D3A的雄蕊明显退化萎缩,且雌蕊发育的速度稍快于D3B,而D3B在整个花蕾发育过程中,雄蕊等花器官均正常发育,且花药鲜黄明亮紧致饱满(图 1E,F).观察花朵可以发现,D3A和D3B均能正常开花,D3A的花瓣大小和形态与D3B没有明显差别,但雄蕊明显退化,D3A的花丝显著短于D3B的花丝,花药干瘪萎缩,无花粉粒释放,而同时期D3B的雄蕊正常发育,花药饱满且可以明显观察到花粉粒的释放(图 1G,H).
-
扫描电镜观察不同时期的雄蕊发现(图 2),在花蕾发育早期,D3A的花药基部开始皱缩,而同时期D3B的花药表现正常(图 2A,D).随着花药继续发育,D3A的花粉囊壁表皮细胞逐渐萎缩,花粉囊开始塌陷,且花药纵径无明显变化;而同时期D3B的花粉囊壁表皮细胞均表现正常,花粉囊持续扩充,花药纵径也明显伸长(图 2B,E).继续观察盛花期的花药发现,D3A的花粉囊完全塌陷萎缩,无花粉粒释放;而D3B的花粉囊始终紧致饱满,花药正常开裂,并释放出大量成熟花粉粒(图 2C,F).
-
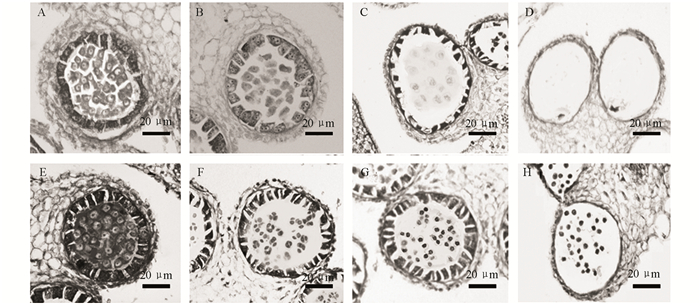
对D3A和D3B不同发育时期的花蕾进行染色制片,比较观察发现在花粉发育早期,D3A与D3B的花粉囊均能正常分化形成周壁组织如表皮层、内皮层、中间层和绒毡层等组织,且均能形成花粉母细胞,但是可以明显观察到D3A的花粉母细胞排列松散,染色较浅,且绒毡层细胞开始呈现出液泡化状态(图 3A);而同时期D3B的花粉母细胞及绒毡层细胞结构完整而饱满,染色较深(图 3E).随着花粉母细胞减数分裂的完成进入四分体时期,在D3A的花粉囊中观察到高度液泡化的绒毡层细胞,而且无四分体结构出现(图 3B);而此时在D3B的花粉囊中可以观察到完整的四分体结构,绒毡层细胞皱缩明显,且细胞质染色较深(图 3F).花药发育后期观察发现,在不育材料D3A的花粉囊中只能看到“拟小抱子”结构逐渐降解消失,因内壁组织不能木质化加厚而不能正常开裂,最终花粉囊内只能观察到残留的“拟小抱子”降解碎片(图 3C,D).此时在对应的可育材料D3B中,随着四分体结构继续发育,可以观察到清晰的单核花粉粒充满花粉囊,随着发育进入到单核花粉粒时期,D3B植株的花粉囊即将开裂释放成熟的花粉粒(图 3G,H).
2.1. 纯合两型系D3A和D3B花发育的体视镜观察
2.2. 纯合两型系D3A和D3B雄蕊扫描电镜分析
2.3. 纯合两型系D3A和D3B花药细胞学观察
-
关于油菜育性的研究,显性核不育主要包括3种不育类型:单基因控制的显性核不育、一对复等位基因控制的显性核不育及双基因控制的显性核不育.本研究中所采用的纯合两型系不育材料D3A和可育材料D3B是经过连续多代姊妹交获得,除了在花期可以观察到花器官的部分差异外,其他田间表型基本保持一致.本研究初步认为这一对材料是基于育性的近等基因系,对不育材料D3A和可育材料D3B的杂交F1代进行育性调查,其分离比例稳定在1:1,且可育株D3B自交后代不会出现育性分离,其遗传表现与单基因显性细胞核雄性不育机理相同[11].
在花期出现花粉败育仅是细胞核雄性不育的表型体现,花粉囊发育的全过程及其分子机理研究是基础和关键.花粉和花药的发育在植物生命过程中至关重要,期间涉及多种基因互作[1-2].傅寿仲等[12]对宜3A进行研究认为,其在花粉母细胞时期前发育正常,在花粉母细胞减数分裂前期出现细胞染色体粘连;后期有的细胞出现染色体桥,在中期Ⅰ,Ⅱ及后期Ⅱ等阶段见到落后染色体. Chowdhury等[13]对黄沙逊和褐沙逊油菜的核不育材料进行细胞学研究,认为在小孢子母细胞减数分裂时都正常,黄沙逊油菜雄性不育的产生在减数分裂后期,由于绒毡层在四分体后期退化,不能起到营养层的作用而导致雄性不育;褐沙逊油菜雄性不育的发生是在减数分裂后期,由于花粉壁细胞不正常地形成了坚实的厚壁引起花药未开裂. Iwasaki等[14]用“Murasaki”核不育油菜为材料进行组织化学检查,结果表明到四分体时期为止,不育株与可育株的花器都还没有差异;四分体时期以后,不育株花药的绒毡层细胞比正常花药的厚而不易破碎,认为绒毡层细胞在花药成熟时期已经没有生理活力,花粉粒不能成熟而导致不育.
在本研究中,对甘蓝型油菜显性细胞核不育纯合两型系不育材料D3A和可育材料D3B不同时期的花蕾进行体视镜观察分析发现,D3A与D3B在外观形态上无明显差别,均能正常开花,且在雄蕊发育早期均表现为正常,后期D3A雄蕊退化萎缩,花粉囊不能正常开裂;不育材料D3A子房柱头发育的速度明显快于D3B.通过对不同时期的花药进行扫描电镜观察发现,D3A早期花药的基部开始皱缩,表皮细胞排列紧密.随着花蕾的发育,D3A花粉囊壁表皮细胞逐渐萎缩,最终塌陷使药室不能正常开裂释放花粉;D3B的花粉囊始终饱满,花药正常开裂,同时释放出大量成熟花粉粒.最后通过石蜡切片观察发现,D3A和D3B在花蕾发育早期都能形成花粉母细胞,但与D3B相比,D3A中的花粉母细胞结构松散,且绒毡层已开始呈现液泡化状态;进入四分体时期,不育材料D3A的花粉囊中观察到高度液泡化的绒毡层细胞,而且无四分体结构出现,表明绒毡层细胞已提前发生降解而未向分泌型转化,使四分体时期小孢子发育丧失了所必需的营养物质,因而不能形成小孢子并发育为成熟的花粉粒进行释放,最终导致不育.因此,本研究认为D3A的败育类型可能属于绒毡层发育异常败育类型.







 DownLoad:
DownLoad: