-
开放科学(资源服务)标识码(OSID):

-
脓毒症(Sepsis)是一种由严重感染引发的全身性炎症反应综合征,可导致多器官功能损伤,并具有较高的病死率[1]。急性呼吸窘迫综合征(Acute Respiratory Distress Syndrome,ARDS)是一种常见且严重的并发症,其特征是由肺内或肺外因素引发的急性、弥漫性炎症性肺损伤,组织学特征包括肺泡水肿、炎症反应、透明膜形成和肺泡出血[2]。脓毒症是引起ARDS的主要原因之一,可通过释放炎症介质,损害全身血管并破坏肺部毛细血管内皮细胞,同时激活肺部免疫细胞,加剧肺损伤,最终导致ARDS的发生[3]。多项研究表明,年龄、吸烟史、感染严重程度以及基础疾病等因素均可能增加脓毒症患者发生ARDS的风险[4-6]。在预测指标方面,APACHEⅡ和SOFA评分已被广泛应用,APACHEⅡ评分通过综合急性生理指标和基础健康状况,从而反映患者的疾病严重程度;SOFA评分则侧重于多器官功能障碍的评估,尤其在脓毒症相关肺损伤的早期识别中具有重要意义[7-8]。此外,一些传统的生物标志物(如C反应蛋白和降钙素原)在脓毒症引发ARDS的预测和识别中也同样具有意义[9]。随着检测技术的进步,研究人员逐渐关注肺上皮损伤标志物的应用潜力,例如表面活性蛋白D(SPD)、晚期糖基化终末产物受体(RAGE)等新兴标志物在预测ARDS风险方面可能具有更高的准确性[10]。鉴于目前尚无统一的危险因素评估标准和风险预测标准,且不同研究间结果差异较大,本研究运用Meta分析系统评价脓毒症并发ARDS(以下简称SA)的发病率、危险因素及相关指标的预测效能,旨在为临床早期干预和个体化治疗提供理论支持。
HTML
-
计算机检索中国知网(以下简称知网)、万方数据知识服务平台(以下简称万方)、维普网(以下简称维普)、Sinomed、PubMed、EMBASE、Web of Science和Cochrane Library数据库,检索日期为2016年1月1日至2024年8月15日。中文主题检索词:脓毒症、脓毒血症、感染性休克、严重脓毒症、全身炎症反应综合征、急性呼吸窘迫综合征、危险因素、发病率、死亡率、患病率、炎症因子、标志物;英文主题检索词:Sepsis、Acute respiratory distress syndrome、Risk factors、Prevalence、Incidence、Mortality、Biomarkers以及相应的自由词。为确保文献的全面性,还检查了纳入研究及综述的参考文献,以搜寻可能遗漏的相关文献。
-
1) 纳入符合Sepsis-3标准的18岁以上非妊娠SA患者(以无ARDS的脓毒症患者作为对照组),且伴随明确诊断为ARDS(符合柏林定义)。
2) 纳入涉及SA的发病率、危险因素或预测指标的观察性研究。
3) 纳入明确报告先发生脓毒症后并发ARDS的研究,排除未明确疾病先后顺序或因其他原因导致ARDS的研究。
4) 发病率(RSA)的计算方法:
式中:NARDS为脓毒症发作后ARDS患者的发生数;N脓毒症为符合诊断标准的脓毒症患者数量。
5) 纳入中英文研究,排除信件、综述、评论、摘要和社论。每项研究均由2名评价员独立评估合格性。评价员首先审阅标题和摘要,随后筛查符合纳入标准的全文。在评价过程中,任何分歧或不确定性均通过讨论协商一致解决。
-
2名评价员单独提取资料并使用预定义的表格评估其质量,任何分歧都通过讨论或咨询第3位评价员来解决。提取的数据包括以下信息:第1作者姓名、地区、研究时间、研究类型、受试者的人口统计学特征、脓毒症例数、SA例数、SA的发病率、危险因素、预测指标。采用纽卡斯尔渥太华质量评估量表(NOS)评估每个队列或病例对照研究的质量。根据NOS评分,研究分为低、中等或高质量,评分为0~3、4~6和7~9。
-
采用Stata SE 15.1软件进行统计分析,使用二分类变量和连续变量的合并比值比(SOR)、标准均值差(SMD)及相应的95%CI来权衡SA的危险因素及预测指标。对分析中涉及的中位数数据,将其转换为均值和标准差[11-12]。采用I2和Q检验评估纳入研究的异质性,显著异质性定义为I2>50%或Q检验p<0.01。当存在显著异质性时,采用随机效应模型;反之,使用固定效应模型。应用亚组分析和Meta回归探讨发病率异质性的潜在来源,采用敏感性分析评估结果的稳健性。采用漏斗图和Egger线性回归评价纳入研究的发表偏倚,不对称漏斗图或Egger检验p<0.05提示存在显著发表偏倚。若检测到显著发表偏倚,进一步使用“剪补法”探讨可能的“缺失研究”对合并效应估计的影响。
1.1. 文献检索策略
1.2. 研究选择
1.3. 数据提取和质量评估
1.4. 统计学分析
-
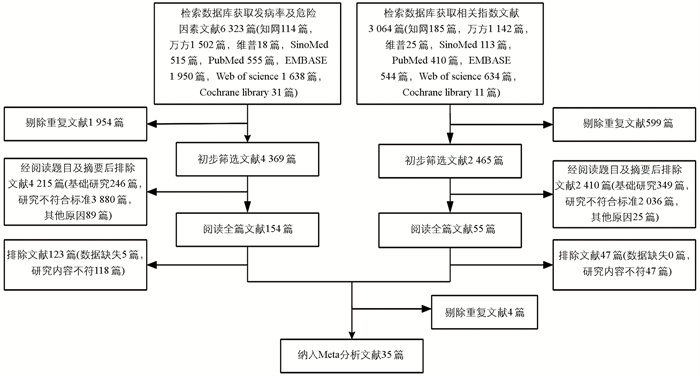
通过检索中英文数据库,分别获取发病率及危险因素相关文献6 323篇记录和相关指标文献3 064篇记录。经过严格筛选,最终纳入35项研究,其中发病率研究4项、危险因素研究12项、相关指标研究4项、发病率和危险因素11项、发病率和危险因素及相关指标研究3项、危险因素和相关指标研究1项(图 1)。
-
35项研究中包含32项队列研究和3项病例对照研究。所有研究发表时间为2017-2024年,脓毒症患者的样本数量为47~2 323人,SA患者共3 715人,涉及研究有亚洲(31条)、欧洲(2条)、北美(2条)地区,所有纳入的研究均被归类为中等至高质量,NOS评分范围为6~9(表 1)。
-
由表 2可知,在18项研究选项中,共纳入4 852例脓毒症患者,其中由脓毒症引起ARDS的患者有402例。ARDS的发病率为9.9%~67.8%,SA的发病率为28.8%,95%CI为0.18~0.40,研究间异质性有统计学意义(I2=98.9%,p<0.01)。
-
通过对SA总发病率的亚组分析(表 2),样本量超过500的选项中发病率相对较高(43.7%),出版年份在2017-2019年选项中发病率相对较低(19.2%);在地域差异方面,欧美的发病率(24.5%)低于亚洲(29.7%)。此外,发病年龄也存在一定的差异,65岁以上人群的发病率(24.9%)低于65岁及以下人群;疾病严重程度APACHEⅡ评分在17分以上时,发病率显著升高(40.3%);SOFA评分为6.5~7.5的发病率也较高(35.7%)。Meta回归分析结果显示,小样本(p=0.016)是影响SA发病率的重要因素。
-
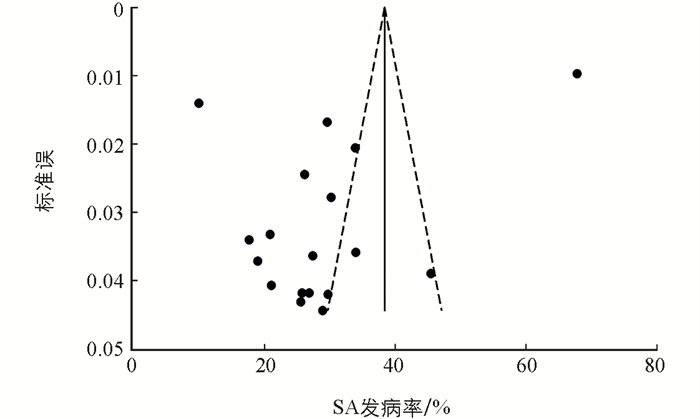
为了探讨个别纳入研究对总体汇总估计的影响,我们进行了敏感性分析。分析结果表明,SA综合发病率与整体合并效应一致,表明总体汇总估计是稳健且可信的。偏倚分析表明,Egger检验(p=0.032)和漏斗图的不对称分布提示了存在小样本效应和发表偏倚的风险。通过修剪填充法的结果表明偏倚对总体效应量的影响较小(图 2)。
-
年龄是SA发病率增加的一个因素(SOR=1.09,95%CI为1.02~1.17),吸烟史提高了SA的发病率(SOR=3.58,95%CI为2.32~5.53),肺部感染显著增加了SA的发病率(SOR=1.97,95%CI为1.68~2.31),合并COPD的患者发病风险显著升高(SOR=6.31,95%CI为3.21~12.41),胰腺炎亦表现出显著的相关性(SOR=3.84,95%CI为2.45~6.02)。此外,休克状态与SA的发病率也显著相关(SOR=1.97,95%CI为1.56~2.49)。在疾病严重程度评分方面,高APACHEⅡ评分与SA发病率升高密切相关(SOR=1.30,95%CI为1.13~1.50),SOFA评分则表现出相关性(SOR=1.23,95%CI为1.11~1.37)。血清乳酸(SOR=1.92,95%CI为1.60~2.32)和血清HMGB1(SOR=2.25,95%CI为1.53~3.31)的高水平也与发病率显著相关。在CRP(SOR=1.01,95%CI为1.00~1.03)、PCT(SOR=1.02,95%CI为0.97~1.06)、IL-6(SOR=1.00,95%CI为0.94~1.07)与SA发病率之间相关性不大(表 3)。
-
对具有显著异质性的肺部感染、休克、APACHEⅡ评分及SOFA评分的危险因素进行了敏感性分析和Egger检验,结果显示肺部感染和休克的合并效应值稳健且与总体结果一致,Egger检验显示研究中存在发表偏倚(p<0.05)。考虑到这一点,我们采用剪补法评估缺失研究对合并结果的影响,分别补充4项研究后,结果并未发生逆转,表明合并结果是稳健的。对于APACHEⅡ评分和SOFA评分,敏感性分析和Egger检验未显示出明显的发表偏倚(p=0.258,p=0.738)。
-
进一步的分析显示,APACHEⅡ评分能够很好地区分脓毒症患者和SA患者,并对ARDS的发生具有预测作用(SMD=2.39,95%CI为0.38~4.40)。我们还发现,SA患者的SOFA评分(SMD=0.74,95%CI为0.47~1.01)、CRP(SMD=0.79,95%CI为0.53~1.04)水平高于脓毒症患者,而PaO2/FiO2(SMD=-1.12,95%CI为-1.33~-0.91)则显著低于脓毒症患者(表 4)。
2.1. 文献筛选结果
2.2. 研究基本特征
2.3. SA发病率Meta分析结果
2.3.1. SA发病率
2.3.2. SA发病率亚组分析
2.3.3. 敏感性分析及发表偏倚检验
2.4. SA危险因素的Meta分析结果
2.4.1. SA危险因素分析结果
2.4.2. 敏感性分析及发表偏倚检验
2.5. SA预测指标的Meta分析结果
-
本研究系统性分析了SA的发病率及危险因素,提供了较为全面的流行病学数据和风险因素特征。总体上,SA的发病率为28.8%,然而研究间的异质性显著,Meta回归分析提示异质性主要源自小样本。在样本量较大的研究中,SA的发病率较高(43.7%),考虑到小样本研究可能因统计效能较低导致发病率的低估,大样本研究覆盖更多患者群体,从而更接近真实水平的发病率。在2017-2019年,SA的发病率为19.2%,这一趋势可能反映出该时期诊疗手段的提升对控制SA的作用[47]。地理差异影响了SA的发病率,欧美地区的发病率为24.5%,低于亚洲的29.7%。有研究表明,欧洲脓毒症的发生率相对别的地区较低,这种差异很可能与当地的种族构成和社会经济水平有关[48]。亚组分析显示,65岁以上人群的SA发病率(24.9%)低于65岁以下人群。这一现象与老年人免疫衰老相关,免疫反应的减弱一定程度上减少了过度炎症的反应[49-50]。
对13个SA危险因素进行分析,最终确定了10个与SA显著相关的危险因素。结果显示,年龄增长被证实为SA的一个显著危险因素,这与早期研究的结论相符[51]。吸烟史、肺部感染、合并COPD、胰腺炎及休克状态均显著增加了SA的患病风险。这些因素可能通过增强炎症反应(如激活中性粒细胞、促炎性细胞因子的释放)和损害肺功能(如肺泡上皮细胞和内皮细胞的破坏、气体交换受限)等机制来促进ARDS的发生[52-53]。此外,APACHEⅡ评分和SOFA评分也被认为是影响SA发病率的重要指标,特别是在APACHEⅡ评分超过17分时,SA的发病率显著上升至40.3%,SOFA评分在6.5~7.5的患者,发病率达到了35.7%。脓毒症患者较高的APACHEⅡ评分和SOFA评分,通常意味着患者的全身炎症反应更为剧烈、器官功能受损更为严重,从而提高了ARDS的发生风险[54]。血清乳酸和血清HMGB1水平升高也与SA有关,这提示了代谢紊乱和炎症在其发病机制中的潜在作用[55]。此外,CRP、PCT和IL-6与SA发病率相关性不大,可能是因为它们更多地反映了全身的炎症状态,未必直接与肺部特异性损伤相关[56],也可能是因为ARDS的发生,除了炎症因子风暴之外,还与氧化应激有关[57]。
在本研究中,我们观察到APACHEⅡ评分(SMD=2.39,95%CI为0.38~4.40)和SOFA评分(SMD=0.74,95%CI为0.47~1.01)在SA预测中具有重要意义。除评分标准外,生物标志物也能对SA进行预测,SA患者的CRP水平显著升高,能够有效区分脓毒症患者和SA患者[58]。这表明CRP作为一个早期指标,对脓毒症患者发生ARDS具有提示作用。考虑到单一指标可能因个体差异而预测能力有限,未来研究应探索将多种生物标志物组合应用于风险评估中,如联合使用RAGE、CXCL16、Ang-2和PaO2/FiO2等指标能很好地预测ARDS的发生风险[25]。这种多个标志物联合模型有助于全面评估疾病的进展,优化早期干预策略。
本研究存在以下局限性:1)纳入的研究存在较大的异质性,尽管我们进行了详细的亚组分析,但多数组别的异质性仍然较高。2)部分预测指标数据较为有限,可能导致预测估计存在不确定性。3)本研究主要纳入了来自亚洲、欧洲和美洲的研究,缺乏其他地区的数据,可能限制了结果的普适性。
综上所述,SA发病率较高,其发生与年龄、吸烟史及多种临床特征密切相关。疾病严重程度评分和炎性标志物对ARDS的发生具有预测作用。未来需要开展更多大样本、跨区域的研究,以探索SA风险因素和预测生物标志物,为早期预示和临床干预提供更可靠的依据。






 DownLoad:
DownLoad: